July 20, 2010 (Atlanta, Georgia) — Cases of H1N1 in cats and ferrets appear to have been transmitted from symptomatic human household members, according to a new analysis from the Oregon Department of Human Services.
Emilio E. DeBess, DVM, from the Oregon Department of Human Services, in Portland, presented the findings here at a poster session at the International Conference on Emerging Infectious Diseases 2010.
"We are reporting the first cluster of laboratory-confirmed cases of H1N1 in the US," the authors note, "in 4 ferrets and 2 cats."
According to Dr. DeBess, the cases represent the first description of the pathology and viral antigen distribution of lethal respiratory disease in domestic cats after natural pandemic (H1N1) 2009 influenza virus infection, which was probably transmitted from humans.
"Pets can be affected by H1N1 by the same means as humans; therefore, patients with H1N1 should also wash their hands and cover their cough to protect their pets," he told Medscape Medical News.
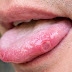
A total of 4 ferrets were infected, presenting with sneezing, coughing, lethargy and nasal discharge. Three of the ferrets had elevated temperatures. In addition, 2 cats affected with H1N1 presented with severe respiratory distress, dyspnea, and cyanosis but did not have an increased temperature. The 2 cats, one a 10-year-old neutered domestic shorthair and the other an 8-year-old spayed domestic shorthair, died shortly after developing severe respiratory disease.
The samples were found to be positive for H1N1 by both the matrix and N1 real-time reverse transcriptase polymer chain reaction assays for the 2009 H1N1 pandemic influenza virus and were subsequently confirmed by the US Department of Agriculture/Animal and Plant Health Inspection Service/Veterinary Services/National Veterinary Services Laboratories.
Marked consolidation of the lung lobes and air bronchograms throughout the chest were observed on x-ray of the cats, and pleural effusion was identified in one. Both cats also exhibited pneumonia and fibrin exudation in bronchioles and alveoli.
According to Dr. DeBess, transmission of H1N1 to pets is the same as it is for humans, but it is unknown at this point whether H1N1 could spread back from pets to humans, although "it could only make sense," he said.
Brett Sponseller, DVM, PhD, assistant professor of vet microbiology and preventive medicine at Iowa State University, in Ames, and colleagues recently reported a similar single case of suspected human-to-cat transmission earlier this year. He commented that the frequency of cross-species transmission to companion animals is unknown at this point. "However, I suspect that it is uncommon, yet underdiagnosed," he told Medscape Medical News. "As in humans, most cases in animals appear to be self-limiting and may go undiagnosed," he added.
According to Dr. Sponseller, with the advent of H1N1, "medical professionals need to be aware of the (reverse) zoonotic potential of this virus and recommend safety precautions to minimize spread to and from companion animals." Recommendations are outlined on the American Veterinary Medical Association Web site.
The authors and commentators have disclosed no relevant financial relationships.
International Conference on Emerging Infectious Diseases (ICEID) 2010: Poster Session. Presented July 13, 2010.
July 22, 2010
July 20, 2010
July 20, 2010 — Trials of vaccines designed to prevent HIV infection might induce antibodies that will cause false-positive results on routine antibody tests for HIV infection. Although the goal of much vaccine development is to induce the production of protective antibodies, they might also cause a state of vaccine-induced seropositivity/reactivity (VISP), which can confound the interpretation of HIV tests in the absence of HIV infection.
In the July 21 issue of JAMA, which focuses on HIV/AIDS, Lindsey Baden, MD, from Brigham and Women's Hospital and Harvard Medical School in Boston, Massachusetts, said that more than 30,000 people have participated in clinical trials of a variety of potential prophylactic vaccines against HIV. These vaccines have used a variety of delivery methods and antigenic compounds and were aimed at inducing different forms of immunity. Dr. Baden discussed the findings at AIDS 2010: XVIII International AIDS Conference in Vienna, Austria.
"The goal of HIV vaccines is to elicit an immune response against HIV. The goal of HIV testing is to see if there is the presence of an immune response to HIV," Dr. Baden explained. "VISP occurs when the antibody or the immune response elicited cross-reacts with the diagnostic test; there can be confusion if one is not aware of this possibility."
VISP might have important ramifications, Dr. Baden said. "Participants may be at risk for misdiagnosis, and if that occurs, then many social harms occur . . . related to insurance, military service, blood [and] tissue donation, immigration, and a variety of other issues that may arise."
Dr. Baden and coworkers studied VISP occurring with various vaccines that were studied by the HIV Vaccine Trials Network. VISP was determined using 3 common US Food and Drug Administration–approved enzyme immunoassay (EIA) test kits. They evaluated VISP in healthy HIV-seronegative adults who participated in any of 25 phase 1 or 2 phase 2a vaccine trials conducted between 2000 and 2010 in the United States and internationally.
VISP was defined as a positive reaction on 1 or more of the EIA tests and a Western blot result that was negative, indeterminate, or atypical-positive — meaning a blot profile consistent with the vaccine product — in conjunction with a negative test for HIV-1 by nucleic acid testing.
Of the 2176 trial participants receiving a test vaccine, 908 (41.7%; 95% confidence interval [CI], 39.6% - 43.8%) had VISP. The occurrence of VISP varied greatly according to the kind of vaccine being tested (e.g., 86.7% for an adenovirus 5 product but only 6.3% for a DNA-alone product). Similarly, results varied substantially according to the test kit used (range, 8.8% to 40.9% VISP).
Dr. Baden concluded that VISP is a common but highly variable outcome in people who participate in vaccine trials. "The occurrence of this is dependent on several factors, including the vaccine or delivery system, the insert used in the vaccine, and the diagnostic test," he said.
These results indicate the need to develop novel rapid-detection methods that do not detect candidate vaccine antigens; several candidate diagnostics are now being investigated that use antigens that are unlikely to be used in vaccines, he noted.
But Dr. Baden said the easiest way to minimize the concern about false-positive test results is for healthcare providers to be aware of the issue as more people participate in vaccine studies. "All they need to do is ask their patients, and if their patients say they are in a vaccine study, then that should be an important consideration in how diagnostic testing is performed," he advised. In addition, testing might best be done by the vaccine trial site, which would be familiar with results related to the specific vaccine.
Because trial participants with VISP might subsequently become infected with HIV, appropriate testing is imperative, Dr. Baden emphasized, including testing for HIV RNA. Trial sites should also test for VISP at the end of the trial and tell participants their VISP status so that they can inform their healthcare providers.
Jason Haukoos, MD, MSc, assistant professor of surgery at the University of Colorado and an emergency physician in the Department of Emergency Medicine at the Denver Health Medical Center, told Medscape Medical News that relatively few patients have been in HIV vaccine trials, so concerns about VISP are small and Dr. Baden's work should go a long way toward solving the problem of false-positive results caused by VISP.
"And there are also a lot of diagnostics coming out now . . . [that will] not only look for antibodies but also antigens," he said. "If you have a combination antibody–antigen assay, then the VISP issue, I think, goes away on some level." He added that, unfortunately, we are still a long way from having an approved vaccine that will be used widely, so the problem of VISP for the near term is minimal.
Melanie Thompson, MD, from the AIDS Research Consortium of Atlanta, Georgia, and chair of the International AIDS Society–USA Antiretroviral Therapy Guidelines Panel, who was not involved in the study, agreed that false-positive HIV test results from VISP are not yet a problem given the small number of trial participants.
"As vaccine studies are expanding, it is going to become a more general issue," she told Medscape Medical News. Patients in vaccine trials will need to be aware of the issue "because they are going to educate the doctors in the community about VISP," she said. "Any HIV testers in the community need to be aware that persons who have been in vaccine trials may have a false-positive HIV test on the antibody testing."
Even in countries with limited healthcare resources, where many of the vaccine trials occur, she said the problem of VISP should be small if the test sites encourage their trial subjects to come back to them for HIV testing, where the proper test methodologies exist, if they have a positive test result elsewhere.
The work was funded by the National Institute of Allergy and Infectious Diseases and by a University of Washington Center for AIDS Research grant. Dr. Baden has disclosed no relevant financial relationships. Dr. Haukoos reports receiving unrestricted research support from Abbott Laboratories and being supported in part by the Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality. Dr. Thompson reports receiving research grants awarded to the AIDS Research Consortium of Atlanta from Abbott Laboratories, Avexa, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, GeoVax, Katketsuken, Koronis Pharmaceuticals, Merck Research Laboratories, Myriad, Ora-Sure, Panacos Pharmaceuticals (now Myriad), Pfizer, Progenics Pharmaceuticals, Roche Laboratories, Roche Molecular Systems, Serono, Theratechnologies Tibotec Therapeutics, Tobira Therapeutics, Trimeris, and VaxGen; serving on the scientific advisory boards or as a clinical trial design consultant for Chimerix, GeoVax, GlaxoSmithKline, Panacos Pharmaceuticals, Progenics Pharmaceuticals, and Tibotec Therapeutics; receiving honoraria for scientific lectures from GlaxoSmithKline and Serono; and serving on data and safety monitoring boards for Tibotec Therapeutics.
JAMA. 2010;304:275-283. Abstract
AIDS 2010: XVIII International AIDS Conference. Presented July 18, 2010.
Continue Reading
| |
| Dr. Lindsey Baden |
"The goal of HIV vaccines is to elicit an immune response against HIV. The goal of HIV testing is to see if there is the presence of an immune response to HIV," Dr. Baden explained. "VISP occurs when the antibody or the immune response elicited cross-reacts with the diagnostic test; there can be confusion if one is not aware of this possibility."
VISP might have important ramifications, Dr. Baden said. "Participants may be at risk for misdiagnosis, and if that occurs, then many social harms occur . . . related to insurance, military service, blood [and] tissue donation, immigration, and a variety of other issues that may arise."
Dr. Baden and coworkers studied VISP occurring with various vaccines that were studied by the HIV Vaccine Trials Network. VISP was determined using 3 common US Food and Drug Administration–approved enzyme immunoassay (EIA) test kits. They evaluated VISP in healthy HIV-seronegative adults who participated in any of 25 phase 1 or 2 phase 2a vaccine trials conducted between 2000 and 2010 in the United States and internationally.
VISP was defined as a positive reaction on 1 or more of the EIA tests and a Western blot result that was negative, indeterminate, or atypical-positive — meaning a blot profile consistent with the vaccine product — in conjunction with a negative test for HIV-1 by nucleic acid testing.
Of the 2176 trial participants receiving a test vaccine, 908 (41.7%; 95% confidence interval [CI], 39.6% - 43.8%) had VISP. The occurrence of VISP varied greatly according to the kind of vaccine being tested (e.g., 86.7% for an adenovirus 5 product but only 6.3% for a DNA-alone product). Similarly, results varied substantially according to the test kit used (range, 8.8% to 40.9% VISP).
Dr. Baden concluded that VISP is a common but highly variable outcome in people who participate in vaccine trials. "The occurrence of this is dependent on several factors, including the vaccine or delivery system, the insert used in the vaccine, and the diagnostic test," he said.
These results indicate the need to develop novel rapid-detection methods that do not detect candidate vaccine antigens; several candidate diagnostics are now being investigated that use antigens that are unlikely to be used in vaccines, he noted.
But Dr. Baden said the easiest way to minimize the concern about false-positive test results is for healthcare providers to be aware of the issue as more people participate in vaccine studies. "All they need to do is ask their patients, and if their patients say they are in a vaccine study, then that should be an important consideration in how diagnostic testing is performed," he advised. In addition, testing might best be done by the vaccine trial site, which would be familiar with results related to the specific vaccine.
Because trial participants with VISP might subsequently become infected with HIV, appropriate testing is imperative, Dr. Baden emphasized, including testing for HIV RNA. Trial sites should also test for VISP at the end of the trial and tell participants their VISP status so that they can inform their healthcare providers.
Jason Haukoos, MD, MSc, assistant professor of surgery at the University of Colorado and an emergency physician in the Department of Emergency Medicine at the Denver Health Medical Center, told Medscape Medical News that relatively few patients have been in HIV vaccine trials, so concerns about VISP are small and Dr. Baden's work should go a long way toward solving the problem of false-positive results caused by VISP.
"And there are also a lot of diagnostics coming out now . . . [that will] not only look for antibodies but also antigens," he said. "If you have a combination antibody–antigen assay, then the VISP issue, I think, goes away on some level." He added that, unfortunately, we are still a long way from having an approved vaccine that will be used widely, so the problem of VISP for the near term is minimal.
Melanie Thompson, MD, from the AIDS Research Consortium of Atlanta, Georgia, and chair of the International AIDS Society–USA Antiretroviral Therapy Guidelines Panel, who was not involved in the study, agreed that false-positive HIV test results from VISP are not yet a problem given the small number of trial participants.
"As vaccine studies are expanding, it is going to become a more general issue," she told Medscape Medical News. Patients in vaccine trials will need to be aware of the issue "because they are going to educate the doctors in the community about VISP," she said. "Any HIV testers in the community need to be aware that persons who have been in vaccine trials may have a false-positive HIV test on the antibody testing."
Even in countries with limited healthcare resources, where many of the vaccine trials occur, she said the problem of VISP should be small if the test sites encourage their trial subjects to come back to them for HIV testing, where the proper test methodologies exist, if they have a positive test result elsewhere.
The work was funded by the National Institute of Allergy and Infectious Diseases and by a University of Washington Center for AIDS Research grant. Dr. Baden has disclosed no relevant financial relationships. Dr. Haukoos reports receiving unrestricted research support from Abbott Laboratories and being supported in part by the Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality. Dr. Thompson reports receiving research grants awarded to the AIDS Research Consortium of Atlanta from Abbott Laboratories, Avexa, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, GeoVax, Katketsuken, Koronis Pharmaceuticals, Merck Research Laboratories, Myriad, Ora-Sure, Panacos Pharmaceuticals (now Myriad), Pfizer, Progenics Pharmaceuticals, Roche Laboratories, Roche Molecular Systems, Serono, Theratechnologies Tibotec Therapeutics, Tobira Therapeutics, Trimeris, and VaxGen; serving on the scientific advisory boards or as a clinical trial design consultant for Chimerix, GeoVax, GlaxoSmithKline, Panacos Pharmaceuticals, Progenics Pharmaceuticals, and Tibotec Therapeutics; receiving honoraria for scientific lectures from GlaxoSmithKline and Serono; and serving on data and safety monitoring boards for Tibotec Therapeutics.
JAMA. 2010;304:275-283. Abstract
AIDS 2010: XVIII International AIDS Conference. Presented July 18, 2010.
July 17, 2010
July 15, 2010 (Winter Park, Florida) — Treatment with the investigational obesity drug lorcaserin (Arena Pharmaceuticals, San Diego, CA) results in significantly greater weight loss than placebo in obese or overweight patients, a new study shows [1]. After one year of treatment, patients treated with the novel weight-loss drug lost 4 kg more than those treated with placebo, with significantly more lorcaserin-treated patients losing more than 5% of their body weight compared with the placebo-treated patients.
"I see the obesity epidemic in the US as a medical issue of pretty amazing proportions when you have one-third of the US population that's obese," lead investigator Dr Steven Smith (Florida Hospital, Winter Park) told heartwire . "If you look at the risk for developing diabetes, cardiovascular disease, and all the way down to orthopedic problems, I think there is a huge unmet need out there for multiple medical conditions . . . a huge unmet need that lorcaserin has the potential to fill."
Importantly, an assessment of adverse events at one and two years did not show any difference in the rates of cardiac valvulopathy, a problem that was observed with less selective obesity agents, report investigators. "In phase 3 studies, particularly in obesity studies, the sample size goes up, which gives us a better picture not just of the efficacy, which I don't think requires many thousands of patients, but lets us really nail some of the safety issues regarding lorcaserin," added Smith.
Lorcaserin is a selective serotonin 2C receptor agonist, and previous studies have shown that drugs targeting serotonin are beneficial in weight loss. The nonselective serotonin agonist fenfluramine, which, along with phentermine, made up the antiobesity medication fen-phen, for example, was approved by the Food and Drug Administration (FDA) in the early 1970s as an adjunct for the treatment of obesity. It was pulled from the market after reports it caused valve disease and pulmonary hypertension.
The results of the study, known as the Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) trial, are published in the July 15, 2010 issue of the New England Journal of Medicine.
Safety of the 5-HT2C Receptor
In BLOOM, 3182 obese or overweight patients--defined as a body-mass index (BMI) between 30 to 45 kg/m2 (or BMI 27 to 45 with at least one preexisting condition, including hypertension, diabetes, cardiovascular disease, impaired glucose tolerance, or sleep apnea)--were randomized to lorcaserin 10 mg twice daily or placebo for 52 weeks. All patients were instructed to exercise moderately for 30 minutes daily and to reduce caloric intake 600 kcal below their estimated daily energy requirements.
At one year, 47.5% of the lorcaserin-treated patients lost 5% or more of their body weight compared with 20.3% of the placebo-treated patients. In addition, 22.7% of the lorcaserin-treated individuals lost 10% of their body weight, while just 7.7% lost the same amount of weight in the placebo arm. On average, individuals treated with lorcaserin lost 5.8 kg compared with 2.2 kg lost in the placebo-treated patients.
After one year of treatment, patients who received lorcaserin were randomly assigned 2:1 to continue treatment for an additional year or to receive placebo. The lorcaserin patients switched to placebo regained the weight lost in the first year and ended year two with roughly the same body weight as those who received placebo for the full two-year study.
"I have thought that for not only lorcaserin, but for other pharmacotherapy approaches, that obesity is a chronic condition, like high blood pressure and hypercholesterolemia," said Smith. "We know that, [just like with other drugs], if you stop taking them, just like you saw here, people regain their body weight. I personally believe that long-term treatment with antiobesity therapies matches the disease and makes a lot of sense, just like treating hypertension."
Asked about the 5.8-kg reduction in weight, Smith told heartwire that for individuals with obesity, every pound lost counts. Medically accepted weight loss, he noted, is approximately 5% of body weight and is generally needed to improve cardiovascular risk factors. Overall, there were small but statistically significant improvements in blood pressure, triglycerides, insulin sensitivity, fibrinogen, and C-reactive protein (CRP), among other measures, compared with placebo. "All the arrows are pointing in the right direction," he said.
In BLOOM, the percentage of patients who remained in the trial at one year was 55.4% in the lorcaserin arm and 45.1% in the placebo arm. The high dropout rate, said Smith, is on par with other antiobesity studies and is likely the result of individuals being able to observe the drug effects by stepping on a scale or tightening their belt buckles, and deciding they no longer needed or wanted to take the drug. He noted that the most frequently reported side effects with lorcaserin were headache, dizziness, and nausea, which occurred early and usually went away. That more people stayed on the drug than on placebo speaks to the tolerability of lorcaserin, said Smith.
Regarding the more serious concern of cardiac valvulopathy, the BLOOM investigators report that at one year FDA-defined valvulopathy developed in 2.3% of patients in the placebo arm and 2.7% of patients in the lorcaserin group, a nonsignificant difference. At two years, the rates were similar.
The Heart Valves
Recent evidence suggests that different serotonin receptors are responsible for different effects, with the 5-HT2B receptor believed responsible for the adverse cardiac valvular effects, while the 5-HT2C receptor targeted by lorcaserin is responsible for weight loss. A smaller 12-week study with lorcaserin showed the drug reduced weight without any adverse effects on the cardiac valves or pulmonary arterial pressure. The Behavioral Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) trial, previously reported by heartwire , showed lorcaserin did not increase the risk of cardiac valvulopathy or worsen valve problems, including in some patients with preexisting mild to greater aortic or mitral regurgitation.
Speaking with heartwire , Dr Frank Greenway (Pennington Biomedical Research Center, Baton Rouge, LA), an obesity expert who does research in obesity drug development but was not affiliated with the BLOOM trial, agreed that most of the concerns with antiobesity drugs stemmed from the lack of specificity with earlier agents.
"Everybody was very excited about fen-phen because it caused a 15% to 20% reduction in weight loss, so after it was taken off the market, the pharmaceutical industry tried to developed a drug specific to the 5-HT2C receptor, which is in the brain and regulates appetite," he said. "Judging from the affinity constants and so forth, it would appear there shouldn't be a problem with heart-valve pathology with lorcaserin because it's a specific 5-HT2C receptor agonist."
The lack of valve problems in a study extended to two years suggests this is no longer a problem, and "the issue has been put to rest," said Greenway.
In an editorial accompany the study [2], Dr Arne Astrup (University of Copenhagen, Denmark) writes that the "history of pharmacologic treatment of obesity is characterized by repetition," with drugs approved and then later yanked because of serious adverse effects detected during postmarketing surveillance.
In addition to fenfluramine, Astrup cites rimonabant, which was scrapped by Sanofi-Aventis after concerns were raised about the drug's psychiatric side effects, and sibutramine (Meridia, Abbott Laboratories), which has been removed from the European market and is contraindicated in individuals with a history of cardiovascular disease. Still, despite these setbacks, Astrup notes that "it makes good sense to develop more selective agents" that work on the 5-HT2C receptors.
However, putting lorcaserin on the market to treat obesity should be based not on the greater efficacy of the drug, which is slightly less than other compounds, but on improved safety and an improved adverse-event profile, as well as meaningful benefits on risk factors for type 2 diabetes mellitus and cardiovascular disease. "Given the history, we will need to be doubly sure about the safety of lorcaserin, used either alone or in combination with other weight-loss drugs," writes Astrup.
Arena Pharmaceuticals sponsored the BLOOM study. Smith reports consulting fees/honorarium from Arena Pharmaceuticals and has received financial support for travel expenses related to the BLOOM study. Smith spoke with heartwire on a conference call set up by Russo Partners, a public-relations firm. Present on the call were David Schull (Russo Partners, New York), Lena Evans (Russo Partners), and Cindy McGee (Arena Pharmaceuticals). Astrup reports receiving grant support from NeuroSearch and Novo Nordisk and currently sits on an external advisory board for Merck and NeuroSearch.
Continue Reading
"I see the obesity epidemic in the US as a medical issue of pretty amazing proportions when you have one-third of the US population that's obese," lead investigator Dr Steven Smith (Florida Hospital, Winter Park) told heartwire . "If you look at the risk for developing diabetes, cardiovascular disease, and all the way down to orthopedic problems, I think there is a huge unmet need out there for multiple medical conditions . . . a huge unmet need that lorcaserin has the potential to fill."
Importantly, an assessment of adverse events at one and two years did not show any difference in the rates of cardiac valvulopathy, a problem that was observed with less selective obesity agents, report investigators. "In phase 3 studies, particularly in obesity studies, the sample size goes up, which gives us a better picture not just of the efficacy, which I don't think requires many thousands of patients, but lets us really nail some of the safety issues regarding lorcaserin," added Smith.
Lorcaserin is a selective serotonin 2C receptor agonist, and previous studies have shown that drugs targeting serotonin are beneficial in weight loss. The nonselective serotonin agonist fenfluramine, which, along with phentermine, made up the antiobesity medication fen-phen, for example, was approved by the Food and Drug Administration (FDA) in the early 1970s as an adjunct for the treatment of obesity. It was pulled from the market after reports it caused valve disease and pulmonary hypertension.
The results of the study, known as the Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) trial, are published in the July 15, 2010 issue of the New England Journal of Medicine.
Safety of the 5-HT2C Receptor
In BLOOM, 3182 obese or overweight patients--defined as a body-mass index (BMI) between 30 to 45 kg/m2 (or BMI 27 to 45 with at least one preexisting condition, including hypertension, diabetes, cardiovascular disease, impaired glucose tolerance, or sleep apnea)--were randomized to lorcaserin 10 mg twice daily or placebo for 52 weeks. All patients were instructed to exercise moderately for 30 minutes daily and to reduce caloric intake 600 kcal below their estimated daily energy requirements.
At one year, 47.5% of the lorcaserin-treated patients lost 5% or more of their body weight compared with 20.3% of the placebo-treated patients. In addition, 22.7% of the lorcaserin-treated individuals lost 10% of their body weight, while just 7.7% lost the same amount of weight in the placebo arm. On average, individuals treated with lorcaserin lost 5.8 kg compared with 2.2 kg lost in the placebo-treated patients.
After one year of treatment, patients who received lorcaserin were randomly assigned 2:1 to continue treatment for an additional year or to receive placebo. The lorcaserin patients switched to placebo regained the weight lost in the first year and ended year two with roughly the same body weight as those who received placebo for the full two-year study.
"I have thought that for not only lorcaserin, but for other pharmacotherapy approaches, that obesity is a chronic condition, like high blood pressure and hypercholesterolemia," said Smith. "We know that, [just like with other drugs], if you stop taking them, just like you saw here, people regain their body weight. I personally believe that long-term treatment with antiobesity therapies matches the disease and makes a lot of sense, just like treating hypertension."
Asked about the 5.8-kg reduction in weight, Smith told heartwire that for individuals with obesity, every pound lost counts. Medically accepted weight loss, he noted, is approximately 5% of body weight and is generally needed to improve cardiovascular risk factors. Overall, there were small but statistically significant improvements in blood pressure, triglycerides, insulin sensitivity, fibrinogen, and C-reactive protein (CRP), among other measures, compared with placebo. "All the arrows are pointing in the right direction," he said.
In BLOOM, the percentage of patients who remained in the trial at one year was 55.4% in the lorcaserin arm and 45.1% in the placebo arm. The high dropout rate, said Smith, is on par with other antiobesity studies and is likely the result of individuals being able to observe the drug effects by stepping on a scale or tightening their belt buckles, and deciding they no longer needed or wanted to take the drug. He noted that the most frequently reported side effects with lorcaserin were headache, dizziness, and nausea, which occurred early and usually went away. That more people stayed on the drug than on placebo speaks to the tolerability of lorcaserin, said Smith.
Regarding the more serious concern of cardiac valvulopathy, the BLOOM investigators report that at one year FDA-defined valvulopathy developed in 2.3% of patients in the placebo arm and 2.7% of patients in the lorcaserin group, a nonsignificant difference. At two years, the rates were similar.
The Heart Valves
Recent evidence suggests that different serotonin receptors are responsible for different effects, with the 5-HT2B receptor believed responsible for the adverse cardiac valvular effects, while the 5-HT2C receptor targeted by lorcaserin is responsible for weight loss. A smaller 12-week study with lorcaserin showed the drug reduced weight without any adverse effects on the cardiac valves or pulmonary arterial pressure. The Behavioral Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) trial, previously reported by heartwire , showed lorcaserin did not increase the risk of cardiac valvulopathy or worsen valve problems, including in some patients with preexisting mild to greater aortic or mitral regurgitation.
Speaking with heartwire , Dr Frank Greenway (Pennington Biomedical Research Center, Baton Rouge, LA), an obesity expert who does research in obesity drug development but was not affiliated with the BLOOM trial, agreed that most of the concerns with antiobesity drugs stemmed from the lack of specificity with earlier agents.
"Everybody was very excited about fen-phen because it caused a 15% to 20% reduction in weight loss, so after it was taken off the market, the pharmaceutical industry tried to developed a drug specific to the 5-HT2C receptor, which is in the brain and regulates appetite," he said. "Judging from the affinity constants and so forth, it would appear there shouldn't be a problem with heart-valve pathology with lorcaserin because it's a specific 5-HT2C receptor agonist."
The lack of valve problems in a study extended to two years suggests this is no longer a problem, and "the issue has been put to rest," said Greenway.
In an editorial accompany the study [2], Dr Arne Astrup (University of Copenhagen, Denmark) writes that the "history of pharmacologic treatment of obesity is characterized by repetition," with drugs approved and then later yanked because of serious adverse effects detected during postmarketing surveillance.
In addition to fenfluramine, Astrup cites rimonabant, which was scrapped by Sanofi-Aventis after concerns were raised about the drug's psychiatric side effects, and sibutramine (Meridia, Abbott Laboratories), which has been removed from the European market and is contraindicated in individuals with a history of cardiovascular disease. Still, despite these setbacks, Astrup notes that "it makes good sense to develop more selective agents" that work on the 5-HT2C receptors.
However, putting lorcaserin on the market to treat obesity should be based not on the greater efficacy of the drug, which is slightly less than other compounds, but on improved safety and an improved adverse-event profile, as well as meaningful benefits on risk factors for type 2 diabetes mellitus and cardiovascular disease. "Given the history, we will need to be doubly sure about the safety of lorcaserin, used either alone or in combination with other weight-loss drugs," writes Astrup.
Arena Pharmaceuticals sponsored the BLOOM study. Smith reports consulting fees/honorarium from Arena Pharmaceuticals and has received financial support for travel expenses related to the BLOOM study. Smith spoke with heartwire on a conference call set up by Russo Partners, a public-relations firm. Present on the call were David Schull (Russo Partners, New York), Lena Evans (Russo Partners), and Cindy McGee (Arena Pharmaceuticals). Astrup reports receiving grant support from NeuroSearch and Novo Nordisk and currently sits on an external advisory board for Merck and NeuroSearch.
July 16, 2010
July 15, 2010 — After reviewing the relevant literature, a panel of neurologists, neuroradiologists, and radiologists has concluded that diffusion-weighted imaging (DWI) MRI is superior to noncontrast computed tomography (CT) scans, which are the current imaging standard, for diagnosing acute ischemic stroke within 12 hours of symptom onset.
However, although the research shows that DWI is better than CT, the decision about which imaging test to use in clinical practice will depend on issues such as availability and cost, the panel concludes in the new guideline from the American Academy of Neurology.
"The doctors taking care of acute stroke patients, as well as the patients themselves, need to be aware that MRI-DWI is a superior diagnostic tool in acute stroke less than 12 hours," lead author Peter Schellinger, MD, PhD, from Johannes Wesling Clinical Center and the University of Erlangen, Germany, told Medscape Medical News.
"Whether this translates into a change in practice remains to be seen," Dr. Schellinger added. "Logistical, financial, and personnel requirements need to be weighed against better diagnosis which — and this was not within the scope of our assessment — may influence management and ultimately the outcome of the stroke."
The panel's recommendations appear in the July 13 issue of Neurology.
For their review, the panel members addressed 2 questions:
The panel classified evidence using a 4-tier classification scheme for diagnostic (question 1) and prognostic (question 2) articles. Recommendations were based on these levels of evidence.
For question 1, the panel identified 62 articles that fulfilled the inclusion criteria. For DWI, there was 1 class I and 3 class II studies. All PWI studies were class IV.
The class I study assessed the accuracy of MRI (DWI and gradient echo scans) vs CT in 356 consecutive patients presenting to a hospital emergency department over 18 months with a possible diagnosis of acute stroke. In the subset of 221 patients scanned within 12 hours of symptom onset, the majority of blinded readers correctly diagnosed acute ischemic stroke by MRI more often than by CT (94 vs 22; P < .0001).
Single Study Justifies Level A Recommendation
"The odds ratio and its 95% confidence interval (CI) of the difference in the proportions was 25 (8-79), indicating an effect size sufficiently large for this single study to justify a Level A recommendation," the authors write. "A similar direction and magnitude of difference were also seen in the subset of 90 patients scanned within 3 hours of onset."
They added that the sensitivity, specificity, and accuracy of DWI in this study were 77%, 96%, and 86% respectively, compared with 16%, 97% and 55% for CT.
One of the class II studies prospectively evaluated 50 patients with ischemic stroke and 4 with transient ischemic attacks. Patients were randomly assigned to receive MRI or CT within 6 hours of stroke onset. The sensitivity of infarct detection by experts blinded to the patients' symptoms but aware that it was an ischemic stroke population was significantly better with DWI (91%) than CT (61%), as was the accuracy (DWI, 91%; CT, 61%).
The sensitivity of DWI for the diagnosis of ischemic stroke in patients with possible stroke is not perfect; the panel found that its "true sensitivity" is probably 80% to 90%.
The panel concluded from this and the other evidence that DWI is superior to CT for the diagnosis of acute ischemic stroke within 12 hours of symptom onset.
For question 2, the panel identified 8 studies fulfilling the inclusion criteria — 4 class IV studies that assessed only the correlation of baseline DWI and PWI lesion volume with chronic lesion volume, 1 class II study that assessed only the correlation of baseline DWI and PWI lesion volume with clinical outcome, and 2 class II studies and 1 class III study that assessed both clinical and morphologic outcomes. None of the studies compared CT with MRI in predicting these outcomes.
From this research, the panel concluded that baseline DWI volume probably predicts baseline clinical stroke severity and final lesion volume in anterior-circulation stroke syndromes and is possibly accurate in predicting clinical outcome. In addition, the evidence showed that baseline DWI volume possibly does not predict the baseline National Institute of Health Stroke Scale score in posterior circulation stroke syndromes.
In addition to its recommendation that DWI should be considered superior to CT for the diagnosis of ischemic stroke in patients presenting within 12 hours of symptom onset, the panel recommended that baseline DWI volume should be considered useful in predicting baseline clinical stroke severity and final lesion volume in anterior-circulation stroke syndromes, but not in predicting baseline National Institute of Health Stroke Scale score in posterior-circulation stroke syndromes.
The panel found there was insufficient evidence to support or refute the value of PWI in diagnosing acute ischemic stroke but said baseline PWI volume may be considered useful in predicting baseline clinical stroke severity. Prospective, well-designed studies are needed to investigate the diagnostic utility of PWI in acute stroke, according to the panel.
When CT Is the Best Diagnostic Tool
Still, there are circumstances under which CT should remain the primary diagnostic tool, said Dr. Schellinger. "Most typically, this would be in comatose patients, patients with contraindications for MRI such as implanted cardiac pacemakers, and in patients who are candidates for intravenous thrombolytic therapy with rt-PA [tissue plasminogen activator]. Here, a CT-based exclusion of intracranial hemorrhage is enough to make a treatment decision."
A plain CT scan is usually performed faster than a multisequence MRI scan, noted Dr. Schellinger. "Loss of time from arrival at the hospital to initiation of thrombolytic therapy is associated with a loss of efficacy and reduction of chance for a good outcome, and potentially also with a higher bleeding risk, and therefore should be avoided by all means."
In situations in which CT was performed first — for example, in a candidate for thrombolysis — and diagnostic uncertainty remains, MRI may be performed in addition to CT after initiation of thrombolytic therapy to optimize diagnostic assessment, added Dr. Schellinger.
A disadvantage of MRI imaging in acute stroke is its relatively high cost. According to Dr. Schellinger, superior technologists often cost more, although this study did not address cost implications of using MRI to diagnose stroke. "Our objective was an assessment; how and whether this is taken as a means to change [emergency department] practice and stroke care practice remains to be seen."
Lack of Availability
Another perceived disadvantage of MRI is its lack of ready availability. MRIs should be as accessible as CT scans in typical hospital settings, said Dr. Schellinger, adding that the technology has been available at all hours in every center he himself has worked in. "Many of the major stroke services in the United States have implemented MRI as an emergency imaging tool. It is a question of dedication and also a question of whether stroke patients should be treated in stroke centers or not."
Until now, noncontrast CT has been the diagnostic standard for acute stroke. "There was nothing else available, and it was clear pretty early that the most important differential diagnosis of acute ischemic stroke — for example, intracranial hemorrhage — can be detected by CT with a close to 100% sensitivity," explained Dr. Schellinger. "By deduction, it is assumed that a clear stroke syndrome that is not caused by hemorrhage likely is caused by ischemic stroke, even if the CT does not show it."
Best Identification of Early Strokes
Approached for a comment, Gary Abrams, MD, professor of neurology at the University of California–San Francisco, said the new guideline is "very timely and important," as therapeutic interventions for acute ischemic stroke continue to advance.
The article validates what most clinicians already know — that DWI is the most effective and sensitive way to diagnose an acute stroke and is superior to CT — said Dr. Abrams. "As we move forward in the future, this may the way that we need to go in terms of best identification of early strokes and identifying the group that's most amenable to treatment."
As well, the guideline begins to address the issue of PWI, added Dr. Abrams. "This is much less widespread in terms of availability and clinical impact, but it's important, and as the authors suggest, the combination of DWI and PWI may turn out to be the most effective way to understand what's going on in terms of diagnosis and prognosis in acute stroke."
Dr. Schellinger has served or serves on scientific advisory boards for Boehringer Ingelheim, ImaRx Therapeutics, Photothera, Cerevast, and CoAxia Inc; has served or serves on speakers' bureaus for and received funding for travel and speaker honoraria from Boehringer Ingelheim, Sanofi, ImaRx Therapeutics, Photothera, Cerevast, CoAxia Inc, Solvay Pharmaceuticals Inc, and GlaxoSmithKline; serves on editorial boards of Stroke and European Neurology; receives royalties from the publication of NeuroIntensiv (Springer, 2008) and received royalties from the publication of Stroke MRI (Steinkopff, 2004); has served as a consultant for CoAxia Inc, Photothera, Cerevast, ImaRx, and Boehringer Ingelheim; and has provided expert testimony, affidavits, and acted as a witness or consultant in legal proceedings. For disclosure information on the other authors, please see the original article.
Neurology. 2010;75:177-185.
Continue Reading
However, although the research shows that DWI is better than CT, the decision about which imaging test to use in clinical practice will depend on issues such as availability and cost, the panel concludes in the new guideline from the American Academy of Neurology.
"The doctors taking care of acute stroke patients, as well as the patients themselves, need to be aware that MRI-DWI is a superior diagnostic tool in acute stroke less than 12 hours," lead author Peter Schellinger, MD, PhD, from Johannes Wesling Clinical Center and the University of Erlangen, Germany, told Medscape Medical News.
"Whether this translates into a change in practice remains to be seen," Dr. Schellinger added. "Logistical, financial, and personnel requirements need to be weighed against better diagnosis which — and this was not within the scope of our assessment — may influence management and ultimately the outcome of the stroke."
The panel's recommendations appear in the July 13 issue of Neurology.
For their review, the panel members addressed 2 questions:
- Are DWI and PWI (perfusion-weighted imaging) sensitive and specific in the diagnosis of acute ischemic stroke compared with concurrent imaging with other techniques, established by follow-up imaging, clinical follow-up, and final discharge diagnosis?
- Does the volume of the DWI or PWI abnormality predict initial clinical severity, final infarct size, and late clinical outcome?
The panel classified evidence using a 4-tier classification scheme for diagnostic (question 1) and prognostic (question 2) articles. Recommendations were based on these levels of evidence.
For question 1, the panel identified 62 articles that fulfilled the inclusion criteria. For DWI, there was 1 class I and 3 class II studies. All PWI studies were class IV.
The class I study assessed the accuracy of MRI (DWI and gradient echo scans) vs CT in 356 consecutive patients presenting to a hospital emergency department over 18 months with a possible diagnosis of acute stroke. In the subset of 221 patients scanned within 12 hours of symptom onset, the majority of blinded readers correctly diagnosed acute ischemic stroke by MRI more often than by CT (94 vs 22; P < .0001).
Single Study Justifies Level A Recommendation
"The odds ratio and its 95% confidence interval (CI) of the difference in the proportions was 25 (8-79), indicating an effect size sufficiently large for this single study to justify a Level A recommendation," the authors write. "A similar direction and magnitude of difference were also seen in the subset of 90 patients scanned within 3 hours of onset."
They added that the sensitivity, specificity, and accuracy of DWI in this study were 77%, 96%, and 86% respectively, compared with 16%, 97% and 55% for CT.
One of the class II studies prospectively evaluated 50 patients with ischemic stroke and 4 with transient ischemic attacks. Patients were randomly assigned to receive MRI or CT within 6 hours of stroke onset. The sensitivity of infarct detection by experts blinded to the patients' symptoms but aware that it was an ischemic stroke population was significantly better with DWI (91%) than CT (61%), as was the accuracy (DWI, 91%; CT, 61%).
The sensitivity of DWI for the diagnosis of ischemic stroke in patients with possible stroke is not perfect; the panel found that its "true sensitivity" is probably 80% to 90%.
The panel concluded from this and the other evidence that DWI is superior to CT for the diagnosis of acute ischemic stroke within 12 hours of symptom onset.
For question 2, the panel identified 8 studies fulfilling the inclusion criteria — 4 class IV studies that assessed only the correlation of baseline DWI and PWI lesion volume with chronic lesion volume, 1 class II study that assessed only the correlation of baseline DWI and PWI lesion volume with clinical outcome, and 2 class II studies and 1 class III study that assessed both clinical and morphologic outcomes. None of the studies compared CT with MRI in predicting these outcomes.
From this research, the panel concluded that baseline DWI volume probably predicts baseline clinical stroke severity and final lesion volume in anterior-circulation stroke syndromes and is possibly accurate in predicting clinical outcome. In addition, the evidence showed that baseline DWI volume possibly does not predict the baseline National Institute of Health Stroke Scale score in posterior circulation stroke syndromes.
In addition to its recommendation that DWI should be considered superior to CT for the diagnosis of ischemic stroke in patients presenting within 12 hours of symptom onset, the panel recommended that baseline DWI volume should be considered useful in predicting baseline clinical stroke severity and final lesion volume in anterior-circulation stroke syndromes, but not in predicting baseline National Institute of Health Stroke Scale score in posterior-circulation stroke syndromes.
The panel found there was insufficient evidence to support or refute the value of PWI in diagnosing acute ischemic stroke but said baseline PWI volume may be considered useful in predicting baseline clinical stroke severity. Prospective, well-designed studies are needed to investigate the diagnostic utility of PWI in acute stroke, according to the panel.
When CT Is the Best Diagnostic Tool
Still, there are circumstances under which CT should remain the primary diagnostic tool, said Dr. Schellinger. "Most typically, this would be in comatose patients, patients with contraindications for MRI such as implanted cardiac pacemakers, and in patients who are candidates for intravenous thrombolytic therapy with rt-PA [tissue plasminogen activator]. Here, a CT-based exclusion of intracranial hemorrhage is enough to make a treatment decision."
A plain CT scan is usually performed faster than a multisequence MRI scan, noted Dr. Schellinger. "Loss of time from arrival at the hospital to initiation of thrombolytic therapy is associated with a loss of efficacy and reduction of chance for a good outcome, and potentially also with a higher bleeding risk, and therefore should be avoided by all means."
In situations in which CT was performed first — for example, in a candidate for thrombolysis — and diagnostic uncertainty remains, MRI may be performed in addition to CT after initiation of thrombolytic therapy to optimize diagnostic assessment, added Dr. Schellinger.
A disadvantage of MRI imaging in acute stroke is its relatively high cost. According to Dr. Schellinger, superior technologists often cost more, although this study did not address cost implications of using MRI to diagnose stroke. "Our objective was an assessment; how and whether this is taken as a means to change [emergency department] practice and stroke care practice remains to be seen."
Lack of Availability
Another perceived disadvantage of MRI is its lack of ready availability. MRIs should be as accessible as CT scans in typical hospital settings, said Dr. Schellinger, adding that the technology has been available at all hours in every center he himself has worked in. "Many of the major stroke services in the United States have implemented MRI as an emergency imaging tool. It is a question of dedication and also a question of whether stroke patients should be treated in stroke centers or not."
Until now, noncontrast CT has been the diagnostic standard for acute stroke. "There was nothing else available, and it was clear pretty early that the most important differential diagnosis of acute ischemic stroke — for example, intracranial hemorrhage — can be detected by CT with a close to 100% sensitivity," explained Dr. Schellinger. "By deduction, it is assumed that a clear stroke syndrome that is not caused by hemorrhage likely is caused by ischemic stroke, even if the CT does not show it."
Best Identification of Early Strokes
Approached for a comment, Gary Abrams, MD, professor of neurology at the University of California–San Francisco, said the new guideline is "very timely and important," as therapeutic interventions for acute ischemic stroke continue to advance.
The article validates what most clinicians already know — that DWI is the most effective and sensitive way to diagnose an acute stroke and is superior to CT — said Dr. Abrams. "As we move forward in the future, this may the way that we need to go in terms of best identification of early strokes and identifying the group that's most amenable to treatment."
As well, the guideline begins to address the issue of PWI, added Dr. Abrams. "This is much less widespread in terms of availability and clinical impact, but it's important, and as the authors suggest, the combination of DWI and PWI may turn out to be the most effective way to understand what's going on in terms of diagnosis and prognosis in acute stroke."
Dr. Schellinger has served or serves on scientific advisory boards for Boehringer Ingelheim, ImaRx Therapeutics, Photothera, Cerevast, and CoAxia Inc; has served or serves on speakers' bureaus for and received funding for travel and speaker honoraria from Boehringer Ingelheim, Sanofi, ImaRx Therapeutics, Photothera, Cerevast, CoAxia Inc, Solvay Pharmaceuticals Inc, and GlaxoSmithKline; serves on editorial boards of Stroke and European Neurology; receives royalties from the publication of NeuroIntensiv (Springer, 2008) and received royalties from the publication of Stroke MRI (Steinkopff, 2004); has served as a consultant for CoAxia Inc, Photothera, Cerevast, ImaRx, and Boehringer Ingelheim; and has provided expert testimony, affidavits, and acted as a witness or consultant in legal proceedings. For disclosure information on the other authors, please see the original article.
Neurology. 2010;75:177-185.
July 12, 2010
July 12, 2010 — Fish oil supplement intake is associated with a lower risk for breast cancer in postmenopausal women, according to the results of the VITamins And Lifestyle (VITAL) Cohort study reported in the July issue of Cancer Epidemiology, Biomarkers & Prevention.
"Use of nonvitamin, nonmineral 'specialty' supplements has increased substantially over recent decades," write Theodore M. Brasky, from Hutchinson Cancer Research Center, University of Washington in Seattle, and colleagues. "Several supplements may have anti-inflammatory or anticancer properties. Additionally, supplements taken for symptoms of menopause have been associated with reduced risk of breast cancer in two case-control studies [but] there have been no prospective studies of the association between the long-term use of these supplements and breast cancer risk."
At baseline in 2000 to 2002, a total of 35,016 postmenopausal women, aged 50 to 76 years and living in western Washington State, completed a 24-page questionnaire concerning their use of specialty supplements. Use was characterized by recency (current vs past), frequency (days/week), and duration (number of years). The Surveillance, Epidemiology, and End Results (SEER) registry showed that from 2000 to 2007, there were 880 incident invasive breast cancers. Cox proportional hazards models allowed estimation of multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs).
For ductal, but not lobular, cancers, current use of fish oil was associated with a reduced risk for breast cancer (HR, 0.68; 95% CI, 0.50 - 0.92), and 10-year average use suggested a trend toward a lower risk (P = .09). Use of the other specialty supplements, including those sometimes taken for menopausal symptoms (black cohosh, dong quai, soy, or St. John's wort) was not associated with breast cancer risk.
"Fish oil may be inversely associated with breast cancer risk," the study authors write. "Fish oil is a potential candidate for chemoprevention studies. Until that time, it is not recommended for individual use for breast cancer prevention."
Limitations of this study include lack of data on supplement dose, reliance on self-report, lack of updated exposure information after baseline, power limited by the relatively low prevalence of use of some specialty supplements, and the possibility of chance findings because 15 specialty supplements were examined.
"[T]his is the first prospective study to report on the association of specialty supplements with breast cancer risk," the study authors conclude. "Our finding of a reduced risk of breast cancer with use of fish oil warrants further study of this agent, focused particularly on timing of exposure and dose, as well as on mechanisms of action that might explain differences by tumor stage or histologic type."
The National Institutes of Health, National Cancer Institute supported this study. The study authors have disclosed no relevant financial relationships.
Cancer Epidemiol Biomarkers Prev. 2010;19:1696-1708. Abstract
Continue Reading
"Use of nonvitamin, nonmineral 'specialty' supplements has increased substantially over recent decades," write Theodore M. Brasky, from Hutchinson Cancer Research Center, University of Washington in Seattle, and colleagues. "Several supplements may have anti-inflammatory or anticancer properties. Additionally, supplements taken for symptoms of menopause have been associated with reduced risk of breast cancer in two case-control studies [but] there have been no prospective studies of the association between the long-term use of these supplements and breast cancer risk."
At baseline in 2000 to 2002, a total of 35,016 postmenopausal women, aged 50 to 76 years and living in western Washington State, completed a 24-page questionnaire concerning their use of specialty supplements. Use was characterized by recency (current vs past), frequency (days/week), and duration (number of years). The Surveillance, Epidemiology, and End Results (SEER) registry showed that from 2000 to 2007, there were 880 incident invasive breast cancers. Cox proportional hazards models allowed estimation of multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs).
For ductal, but not lobular, cancers, current use of fish oil was associated with a reduced risk for breast cancer (HR, 0.68; 95% CI, 0.50 - 0.92), and 10-year average use suggested a trend toward a lower risk (P = .09). Use of the other specialty supplements, including those sometimes taken for menopausal symptoms (black cohosh, dong quai, soy, or St. John's wort) was not associated with breast cancer risk.
"Fish oil may be inversely associated with breast cancer risk," the study authors write. "Fish oil is a potential candidate for chemoprevention studies. Until that time, it is not recommended for individual use for breast cancer prevention."
Limitations of this study include lack of data on supplement dose, reliance on self-report, lack of updated exposure information after baseline, power limited by the relatively low prevalence of use of some specialty supplements, and the possibility of chance findings because 15 specialty supplements were examined.
"[T]his is the first prospective study to report on the association of specialty supplements with breast cancer risk," the study authors conclude. "Our finding of a reduced risk of breast cancer with use of fish oil warrants further study of this agent, focused particularly on timing of exposure and dose, as well as on mechanisms of action that might explain differences by tumor stage or histologic type."
The National Institutes of Health, National Cancer Institute supported this study. The study authors have disclosed no relevant financial relationships.
Cancer Epidemiol Biomarkers Prev. 2010;19:1696-1708. Abstract
July 10, 2010
July 2, 2010 — Children who experience serious adversities are at a greater risk for the onset and persistence of suicidal behavior throughout life, according to a new multicountry survey study.
Although exposure to many different adversities "are powerful predictors" for suicidal behavior in later life, sexual or physical abuse during childhood are the strongest risk factors of all, write lead study author Ronny Bruffaerts, PhD, associate professor of psychiatry in the Department of Neurosciences at Katholieke Universiteit Leuven in Belgium, and colleagues.
"Even after rigorously controlling for a broad set of variables, there was at least a threefold increase in lifetime suicide attempt and lifetime suicide ideation among individuals with a history of sexual or physical abuse," they note.
"The point is that childhood trauma has a systematic strong predictive value towards both worse mental and somatic health, as well as increased suicidal behavior," Dr. Bruffaerts told Medscape Medical News.
"Identifying those families at risk of problems, and offering help, may be a way of decreasing suicide around the world," he added in a statement.
The study is published in the July issue of the British Journal of Psychiatry.
Worldwide Suicide Rate Increasing
"Suicides are still one of the major causes of death worldwide," said Dr. Bruffaerts. He added that a report from the World Health Organization showed that the worldwide suicide rate has been increasing steadily since the 1950s.
"Most research on predictors of suicidal behavior focuses on mental disorders as main risk factors, but in the past years there have been some important findings that linked adversities with suicidality," he noted. Also, "the field of childhood adversities is especially an important field to study because its long-term effects have not been studied extensively."
For this study, the investigators evaluated data from nationally representative samples from the World Mental Health surveys. A total of 55,299 people from 21 countries in Africa, the Americas, Asia and the Pacific, Europe, and the Middle East were included.
All participants were interviewed in person about their childhood and whether they had experienced any of the following before they turned 18 years of age: physical abuse, sexual abuse, neglect, parental death, parent divorce, other parental loss, family violence, physical illness, and financial adversity.
Core diagnostic assessments of mental disorders were also made at that time, and the Composite International Diagnostic Interview 3.0 was used to assess lifetime suicidal behavior.
By using these surveys, "we were able to check whether this association [between adversities and suicidal behaviors] held for different countries, different cultures, and different contexts," said Dr. Bruffaerts.
Strong Associations Found
Results showed that 12.2% of the study participants had experienced the death of a parent, 8% had been the victim of physical abuse, and 6.9% had experienced family violence.
A total of 2.7% reported at least 1 suicide attempt, and 9.4% said they had thought about killing themselves.
Among those who had tried to kill themselves, 29.3% had been the victim of physical abuse, 24.8% had experienced family violence, and 14.5% had been sexually abused.
In both bivariate and multivariate models, the childhood adversities were associated with an increased risk for suicide attempt and ideation (odd ratio [OR] range, 1.2 – 5.7) and "the risk increased with the number of adversities experienced, but at a decreasing rate," report the study authors.
In the bivariate models, physical and sexual abuse had the highest odds for suicide attempts (OR, 3.7 and 5.7, respectively) and for suicide ideation (OR, 2.7 and 3.4, respectively). In multivariate additive models, "odds ratios decreased but none lost their statistical significance," the study authors write.
In addition, "associations remained similar after additional adjustment for respondents' lifetime mental disorder status," they add.
Finally, significantly strong associations were found between childhood adversities and suicide attempts in childhood (median OR, 3.8). These associations decreased during the teen years (median OR, 2.5) and in young adulthood (median OR, 2.0) before increasing again in later adulthood (median OR, 2.3).
"Specifically, a history of childhood sexual abuse was associated with a 10.9-fold increase in the odds of a [suicide] attempt between the ages of 4 and 12 years, a 6.1-fold increase in the odds of an attempt between the ages of 13 and 19 years, and a 2.9-fold increase among those between the ages of 20 and 29 years," write the study authors.
Associations Valid Worldwide
The study's overall finding of a strong association between childhood adversities and suicidal behaviors "was not really surprising because prior studies have also shown this," said Dr. Bruffaerts.
However, he noted that "important new findings" included the lifelong effects of childhood adversities, that their highest impact was in childhood and teen years, and that "bodily intrusive adversities" had a stronger impact than other events. But again, "this was not really surprising because it fits with impressions clinicians have."
"That we were able to show that the associations are valid for general populations worldwide was a major step ahead," added Dr. Bruffaerts. "I was [also] surprised...how prevalent childhood adversities actually are in the general population."
When asked about his plans for future research, Dr. Bruffaerts said that a valuable next step pertains to the question, "How can we change the suicidal process?"
"We know that the suicidal process is a treatable condition, but we don’t know exactly how many suicidal persons actually receive treatment," he explained. "In general, I think it is quintessential to generate prevalence estimates of mental disorders worldwide, their correlates and predictors, [and] the ways and why people with psychological problems seek help or not.
"In my opinion, this is an important way to gather data to build national and country-specific policies on," concluded Dr. Bruffaerts.
This study was funded by the US National Institute of Mental Health, the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the US Public Health Service, the Fogarty International Center, the Pan American Health Organization, the Eli Lilly & Company Foundation, Ortho-McNeil Pharmaceutical, GlaxoSmithKline, and Bristol-Myers Squibb. A list of all funders for the various countries' individual health surveys can be found in the original article. Dr. Bruffaerts and all but one of the study authors have disclosed no relevant financial relationships. Investigational team member Ronald C. Kessler reported several declarations of interest, which are listed in full in the original article.
Br J Psychiatry. 2010;197:20-27.
Continue Reading
Although exposure to many different adversities "are powerful predictors" for suicidal behavior in later life, sexual or physical abuse during childhood are the strongest risk factors of all, write lead study author Ronny Bruffaerts, PhD, associate professor of psychiatry in the Department of Neurosciences at Katholieke Universiteit Leuven in Belgium, and colleagues.
"Even after rigorously controlling for a broad set of variables, there was at least a threefold increase in lifetime suicide attempt and lifetime suicide ideation among individuals with a history of sexual or physical abuse," they note.
"The point is that childhood trauma has a systematic strong predictive value towards both worse mental and somatic health, as well as increased suicidal behavior," Dr. Bruffaerts told Medscape Medical News.
"Identifying those families at risk of problems, and offering help, may be a way of decreasing suicide around the world," he added in a statement.
The study is published in the July issue of the British Journal of Psychiatry.
Worldwide Suicide Rate Increasing
"Suicides are still one of the major causes of death worldwide," said Dr. Bruffaerts. He added that a report from the World Health Organization showed that the worldwide suicide rate has been increasing steadily since the 1950s.
"Most research on predictors of suicidal behavior focuses on mental disorders as main risk factors, but in the past years there have been some important findings that linked adversities with suicidality," he noted. Also, "the field of childhood adversities is especially an important field to study because its long-term effects have not been studied extensively."
For this study, the investigators evaluated data from nationally representative samples from the World Mental Health surveys. A total of 55,299 people from 21 countries in Africa, the Americas, Asia and the Pacific, Europe, and the Middle East were included.
All participants were interviewed in person about their childhood and whether they had experienced any of the following before they turned 18 years of age: physical abuse, sexual abuse, neglect, parental death, parent divorce, other parental loss, family violence, physical illness, and financial adversity.
Core diagnostic assessments of mental disorders were also made at that time, and the Composite International Diagnostic Interview 3.0 was used to assess lifetime suicidal behavior.
By using these surveys, "we were able to check whether this association [between adversities and suicidal behaviors] held for different countries, different cultures, and different contexts," said Dr. Bruffaerts.
Strong Associations Found
Results showed that 12.2% of the study participants had experienced the death of a parent, 8% had been the victim of physical abuse, and 6.9% had experienced family violence.
A total of 2.7% reported at least 1 suicide attempt, and 9.4% said they had thought about killing themselves.
Among those who had tried to kill themselves, 29.3% had been the victim of physical abuse, 24.8% had experienced family violence, and 14.5% had been sexually abused.
In both bivariate and multivariate models, the childhood adversities were associated with an increased risk for suicide attempt and ideation (odd ratio [OR] range, 1.2 – 5.7) and "the risk increased with the number of adversities experienced, but at a decreasing rate," report the study authors.
In the bivariate models, physical and sexual abuse had the highest odds for suicide attempts (OR, 3.7 and 5.7, respectively) and for suicide ideation (OR, 2.7 and 3.4, respectively). In multivariate additive models, "odds ratios decreased but none lost their statistical significance," the study authors write.
In addition, "associations remained similar after additional adjustment for respondents' lifetime mental disorder status," they add.
Finally, significantly strong associations were found between childhood adversities and suicide attempts in childhood (median OR, 3.8). These associations decreased during the teen years (median OR, 2.5) and in young adulthood (median OR, 2.0) before increasing again in later adulthood (median OR, 2.3).
"Specifically, a history of childhood sexual abuse was associated with a 10.9-fold increase in the odds of a [suicide] attempt between the ages of 4 and 12 years, a 6.1-fold increase in the odds of an attempt between the ages of 13 and 19 years, and a 2.9-fold increase among those between the ages of 20 and 29 years," write the study authors.
Associations Valid Worldwide
The study's overall finding of a strong association between childhood adversities and suicidal behaviors "was not really surprising because prior studies have also shown this," said Dr. Bruffaerts.
However, he noted that "important new findings" included the lifelong effects of childhood adversities, that their highest impact was in childhood and teen years, and that "bodily intrusive adversities" had a stronger impact than other events. But again, "this was not really surprising because it fits with impressions clinicians have."
"That we were able to show that the associations are valid for general populations worldwide was a major step ahead," added Dr. Bruffaerts. "I was [also] surprised...how prevalent childhood adversities actually are in the general population."
When asked about his plans for future research, Dr. Bruffaerts said that a valuable next step pertains to the question, "How can we change the suicidal process?"
"We know that the suicidal process is a treatable condition, but we don’t know exactly how many suicidal persons actually receive treatment," he explained. "In general, I think it is quintessential to generate prevalence estimates of mental disorders worldwide, their correlates and predictors, [and] the ways and why people with psychological problems seek help or not.
"In my opinion, this is an important way to gather data to build national and country-specific policies on," concluded Dr. Bruffaerts.
This study was funded by the US National Institute of Mental Health, the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the US Public Health Service, the Fogarty International Center, the Pan American Health Organization, the Eli Lilly & Company Foundation, Ortho-McNeil Pharmaceutical, GlaxoSmithKline, and Bristol-Myers Squibb. A list of all funders for the various countries' individual health surveys can be found in the original article. Dr. Bruffaerts and all but one of the study authors have disclosed no relevant financial relationships. Investigational team member Ronald C. Kessler reported several declarations of interest, which are listed in full in the original article.
Br J Psychiatry. 2010;197:20-27.
June 21, 2010
June 16, 2010 (Chicago, Illinois) — In contrast to past advice to cancer patients to rest and avoid activity, the message now is to avoid inactivity. An expert panel convened by the American College of Sports Medicine (ACSM) has concluded that exercise training is safe during and after cancer treatments and can improve physical functioning, quality of life, and cancer-related fatigue.
The new ACSM guidelines urge cancer patients to be as physically active as possible both during and after treatment.
"The take-home message from the panel that put together the guidelines is to avoid inactivity during and posttreatment," said Kathryn Schmitz, PhD, MPH, associate professor of epidemiology and biostatistics at the University of Pennsylvania School of Medicine in Philadelphia. She presented the guidelines here at the American Society of Clinical Oncology 2010 Annual Meeting.
"Dozens of randomized controlled trials in a broad variety of patient populations have established the safety of exercise during treatment and the ability to go from being sedentary to completing 150 minutes of aerobic active over the course of even a single month," she said. "The risk–benefit leans heavily in the direction of getting patients moving and keeping them moving."
Exercise Oncology
Exercise is an area that is gaining an increasing awareness in the cancer literature, noted Jennifer A. Ligibel, MD, who moderated the session where the guidelines were presented. Dr. Ligibel is from the Dana-Farber Cancer Center in Boston, Massachusetts.
"If you had done a search between 1950 and 1979 using the words 'exercise/physical activity' and 'cancer,' you would have found 12 references," said Dr. Ligibel. "But in 2009, there were almost 500, more than almost all of the years put together."
A growing number of reports have now shown that physically active individuals are less likely to develop a number of common cancers, including breast, colon, advanced prostate, some gynecologic cancers, she pointed out. "Evidence is mounting and consistent. There has been a growing number of reports that physically active individuals tend to do better after being diagnosed with cancer."
Much of the data, she pointed out, have been in breast cancer.
Even with a growing number of reports, exercise oncology is a field that is still in its infancy, explained Lee Jones, PhD, scientific director of the Duke Center for Cancer Survivorship at Duke University in Durham, North Carolina. "There are only about 80 or so studies focused on exercise in cancer, and that really isn't all that many, especially when compared to cardiovascular disease — where there are about 3500."
Evidence So Far Is "Very Promising"
Dr. Jones, who was approached by Medscape Oncology for independent comment, pointed out that the evidence thus far is very promising. "Higher levels of physical activity are associated with a significant risk reduction in mortality in cancer patients," he said. "And the research to date provides a platform to launch second-generation studies."
The next level of studies will need to focus on why exercise is an effective intervention in cancer patients, if it does have an effect on recurrence, and if so, the underlying mechanism, Dr. Jones explained.
It is hoped that future research will answer such questions as how the effect can be maximized, what intensity is needed, and if exercise is effective for some types of tumors and not others, he said.
The ACSM guidelines indicate that exercise is safe during treatment, but Dr. Jones acknowledges that that can be a difficult time for patients. "They are battling many things during that time, such as side effects, and exercise might be a little tougher," he said. "But exercise programs can be modulated to suit the patient; that is the premise of personalized medicine."
An exercise program is also dependent on where the patient was in life before their diagnosis. "Were they sedentary, athletic, exercising occasionally?" he asked. "That information is important in shaping an exercise program."
Dr. Jones stated that he would like to get to the point where exercise becomes part of the standard of care for cancer. "It may eventually reach the same standard as it is in cardiac disease," he said. "I see us building this evidence base."
Specific Risks Need to Be Addressed
In creating the guidelines, the authors focused on the adult cancers and sites (breast, prostate, hematologic, colon, and gynecologic) where the most evidence has been assembled, and reviewed the literature for multiple health outcomes.
The panel found that even though there are specific risks associated with cancer treatment that need to be considered when patients embark on an exercise program, the evidence is consistent that it can improve aerobic fitness, muscular strength, quality of life, and fatigue in cancer survivors.
There are some general medical assessments that are recommended prior to exercise, explained Dr. Schmitz. For example, an evaluation for peripheral neuropathies and musculoskeletal morbidities secondary to treatment is recommended, regardless of the time since treatment.
"This doesn't have to be done in the physician's office or by a nurse," she said. "Fitness professionals can ask if there are any changes in balance or whether the person has noticed any tingling or changes in the kinds of symptoms that go along with peripheral neuropathies."
Evaluations of fracture risk are also recommended for individuals who have undergone hormonal therapy, and those with known metastatic disease to the bone will require evaluation to discern what is safe before starting exercise, she added. "Patients with metastatic disease to the bone should have medical clearance before starting an exercise program."
Individuals with known cardiac conditions, whether secondary to cancer or not, also require a medical assessment before starting exercise.
"There is recognition that there is always a risk that metastasis to the bone or cardiac toxicity secondary to cancer treatments will be undetected in a portion of our 12 million cancer survivors out there," Dr. Schmitz said. "This risk will vary widely across the population of survivors, and is likely quite low in the great majority of people who are diagnosed at an early stage and grade."
It is therefore recommended that fitness professionals consult with the patient's medical team to determine the likelihood of this risk, she added. "What we concluded as a body, in order to reduce barriers to physical activity programs, is that requiring medical assessment for metastatic disease and cardiotoxicity for all survivors prior to exercise is not recommended."
"We chose to do this because we felt that the small risk in a small body of patients is probably less than the risk of telling patients that they shouldn't exercise until they are cleared," she explained. "The risk of inactivity for the great majority of people at low risk is greater than the small risk of putting someone in harm's way."
Research Gaps Remain
The panel found that there is consistent evidence that exercise training can lead to improvements in aerobic fitness, muscular strength, quality of life, and fatigue in breast, prostate, and hematologic cancer patients and survivors, but that the data for colon and gynecologic cancers are still too limited to lead to conclusions.
Multiple research gaps remain in this field, the panel notes. These include a need for greater specificity with regard to the dose-response effects of specific modes of exercise training on specific end points and within a broader range of populations, such as survivors of colon and gynecologic cancers.
They also urge fitness trainers who work with cancer survivors to learn as much as possible about the specifics of the cancer diagnosis and treatment to make informed safe choices with regard to exercise testing and prescription.
Cancer diagnosis and treatment effects numerous body systems that are required for and affected by exercise training, they conclude, and "because cancer treatments are increasingly customized according to specific tumor characteristics, fitness professionals may benefit from contacting the medical treatment team for more precise information regarding the treatments received."
Dr. Schmitz and Dr. Ligibel have disclosed no relevant financial relationships.
American Society of Clinical Oncology (ASCO) 2010 Annual Meeting: Presented June 6, 2010.
Continue Reading
The new ACSM guidelines urge cancer patients to be as physically active as possible both during and after treatment.
"The take-home message from the panel that put together the guidelines is to avoid inactivity during and posttreatment," said Kathryn Schmitz, PhD, MPH, associate professor of epidemiology and biostatistics at the University of Pennsylvania School of Medicine in Philadelphia. She presented the guidelines here at the American Society of Clinical Oncology 2010 Annual Meeting.
"Dozens of randomized controlled trials in a broad variety of patient populations have established the safety of exercise during treatment and the ability to go from being sedentary to completing 150 minutes of aerobic active over the course of even a single month," she said. "The risk–benefit leans heavily in the direction of getting patients moving and keeping them moving."
Exercise Oncology
Exercise is an area that is gaining an increasing awareness in the cancer literature, noted Jennifer A. Ligibel, MD, who moderated the session where the guidelines were presented. Dr. Ligibel is from the Dana-Farber Cancer Center in Boston, Massachusetts.
"If you had done a search between 1950 and 1979 using the words 'exercise/physical activity' and 'cancer,' you would have found 12 references," said Dr. Ligibel. "But in 2009, there were almost 500, more than almost all of the years put together."
A growing number of reports have now shown that physically active individuals are less likely to develop a number of common cancers, including breast, colon, advanced prostate, some gynecologic cancers, she pointed out. "Evidence is mounting and consistent. There has been a growing number of reports that physically active individuals tend to do better after being diagnosed with cancer."
Much of the data, she pointed out, have been in breast cancer.
Even with a growing number of reports, exercise oncology is a field that is still in its infancy, explained Lee Jones, PhD, scientific director of the Duke Center for Cancer Survivorship at Duke University in Durham, North Carolina. "There are only about 80 or so studies focused on exercise in cancer, and that really isn't all that many, especially when compared to cardiovascular disease — where there are about 3500."
Evidence So Far Is "Very Promising"
Dr. Jones, who was approached by Medscape Oncology for independent comment, pointed out that the evidence thus far is very promising. "Higher levels of physical activity are associated with a significant risk reduction in mortality in cancer patients," he said. "And the research to date provides a platform to launch second-generation studies."
The next level of studies will need to focus on why exercise is an effective intervention in cancer patients, if it does have an effect on recurrence, and if so, the underlying mechanism, Dr. Jones explained.
It is hoped that future research will answer such questions as how the effect can be maximized, what intensity is needed, and if exercise is effective for some types of tumors and not others, he said.
The ACSM guidelines indicate that exercise is safe during treatment, but Dr. Jones acknowledges that that can be a difficult time for patients. "They are battling many things during that time, such as side effects, and exercise might be a little tougher," he said. "But exercise programs can be modulated to suit the patient; that is the premise of personalized medicine."
An exercise program is also dependent on where the patient was in life before their diagnosis. "Were they sedentary, athletic, exercising occasionally?" he asked. "That information is important in shaping an exercise program."
Dr. Jones stated that he would like to get to the point where exercise becomes part of the standard of care for cancer. "It may eventually reach the same standard as it is in cardiac disease," he said. "I see us building this evidence base."
Specific Risks Need to Be Addressed
In creating the guidelines, the authors focused on the adult cancers and sites (breast, prostate, hematologic, colon, and gynecologic) where the most evidence has been assembled, and reviewed the literature for multiple health outcomes.
The panel found that even though there are specific risks associated with cancer treatment that need to be considered when patients embark on an exercise program, the evidence is consistent that it can improve aerobic fitness, muscular strength, quality of life, and fatigue in cancer survivors.
There are some general medical assessments that are recommended prior to exercise, explained Dr. Schmitz. For example, an evaluation for peripheral neuropathies and musculoskeletal morbidities secondary to treatment is recommended, regardless of the time since treatment.
"This doesn't have to be done in the physician's office or by a nurse," she said. "Fitness professionals can ask if there are any changes in balance or whether the person has noticed any tingling or changes in the kinds of symptoms that go along with peripheral neuropathies."
Evaluations of fracture risk are also recommended for individuals who have undergone hormonal therapy, and those with known metastatic disease to the bone will require evaluation to discern what is safe before starting exercise, she added. "Patients with metastatic disease to the bone should have medical clearance before starting an exercise program."
Individuals with known cardiac conditions, whether secondary to cancer or not, also require a medical assessment before starting exercise.
"There is recognition that there is always a risk that metastasis to the bone or cardiac toxicity secondary to cancer treatments will be undetected in a portion of our 12 million cancer survivors out there," Dr. Schmitz said. "This risk will vary widely across the population of survivors, and is likely quite low in the great majority of people who are diagnosed at an early stage and grade."
It is therefore recommended that fitness professionals consult with the patient's medical team to determine the likelihood of this risk, she added. "What we concluded as a body, in order to reduce barriers to physical activity programs, is that requiring medical assessment for metastatic disease and cardiotoxicity for all survivors prior to exercise is not recommended."
"We chose to do this because we felt that the small risk in a small body of patients is probably less than the risk of telling patients that they shouldn't exercise until they are cleared," she explained. "The risk of inactivity for the great majority of people at low risk is greater than the small risk of putting someone in harm's way."
Research Gaps Remain
The panel found that there is consistent evidence that exercise training can lead to improvements in aerobic fitness, muscular strength, quality of life, and fatigue in breast, prostate, and hematologic cancer patients and survivors, but that the data for colon and gynecologic cancers are still too limited to lead to conclusions.
Multiple research gaps remain in this field, the panel notes. These include a need for greater specificity with regard to the dose-response effects of specific modes of exercise training on specific end points and within a broader range of populations, such as survivors of colon and gynecologic cancers.
They also urge fitness trainers who work with cancer survivors to learn as much as possible about the specifics of the cancer diagnosis and treatment to make informed safe choices with regard to exercise testing and prescription.
Cancer diagnosis and treatment effects numerous body systems that are required for and affected by exercise training, they conclude, and "because cancer treatments are increasingly customized according to specific tumor characteristics, fitness professionals may benefit from contacting the medical treatment team for more precise information regarding the treatments received."
Dr. Schmitz and Dr. Ligibel have disclosed no relevant financial relationships.
American Society of Clinical Oncology (ASCO) 2010 Annual Meeting: Presented June 6, 2010.
June 17, 2010 — Vitamin D may reduce the incidence and severity of influenza and other infections of the upper respiratory tract, new research indicates.
Simple steps such as eating foods rich with vitamin D and getting more sunshine may help to reduce your chances of contracting flu and other similar illnesses, shows a study by scientists at Yale University School of Medicine and Greenwich Hospital in Connecticut.
"People in the South and West get more sun than in the North, which is good for them, because you get vitamin D from the sun," study researcher James R. Sabetta, MD, of the Yale University School of Medicine and Greenwich Hospital, Conn., tells WebMD. "It's not a panacea, but it helps."
Sabetta and his team of colleagues followed 198 healthy adults during the fall and winter of 2009-2010 to see if declining levels of vitamin D in the fall and winter could be a factor in the seasonal increased prevalence of respiratory viral infections, such as flu.
The study shows people who maintain vitamin D blood levels of 38 nanograms per milliliter or more are less likely to get viral infections such as flu than people with less in their blood.
Of 18 people who maintained that level during the study period, only three developed viral infections.
But of the 180 other participants with less vitamin D in their blood, 81(45%), did get sick with viral infections.
And those with higher levels of vitamin D also experienced a marked reduction in the number of days they were ill, Sabetta tells WebMD.
In addition to getting more sun and consuming milk and foods with vitamin D, he recommends supplements, especially for people in areas with less sunlight and for those who spend daylight hours in darker, indoor environments.
"If you have a level of 38, your risk is down 50%," he tells WebMD. "A lot of people don't have an adequate level, and 38 is a little above what you should have to be considered in the sufficient range. There are a billion people worldwide with levels below 30."
And 30 is considered "sufficient," he says.
Watching for Signs of Illness
Participants in the study had blood samples drawn monthly using a sophisticated technique to accurately measure vitamin D levels. They didn't know that vitamin D was being measured, and even investigators didn't know until the end of the study.
All participants were asked to report signs of illness, such as nasal congestion, sore throat, cough with or without fever, chills, fatigue and general malaise.
Those reporting any symptoms were seen the same day at the study site by one of the infectious disease investigators.
People in the study kept a diary of symptoms and were called every one to three days during the illness to review any signs of symptoms until they were better. The investigators recorded the duration of each symptom, the total duration of the illness, and any antimicrobials administered.
Sabetta says the findings suggest that supplementing vitamin D to achieve a blood level of 38 nanograms per milliliter or higher could result in a significant health benefit by reducing odds of contracting viral infections of the respiratory tract.
But he says more studies are needed to determine the efficacy of vitamin D supplementation in the prevention of infections, including influenza.
The researchers conclude that the lower levels of vitamin D seen during the winter in temperate climates may contribute to the prevalence of influenza in colder months.
The findings, Sabetta says, have significant implications for public health and also may explain the seasonality of certain infections, and also the higher morbidity and mortality of such illnesses in people who are predisposed to lower concentrations of vitamin D.
Sabetta says vitamin D has known effects on the immune system, and the study reinforces the association between vitamin D deficiency and susceptibility to infections of the respiratory tract.
The study is published online in the journal Plos ONE.
Vitamin D levels depend "on how big you are, your skin color, your diet and how much sun exposure you get," Sabetta tells WebMD. "Individuals should get their vitamin D levels checked. If you are gardening a lot, you probably are fine, but people in an office all day may need supplements."
SOURCES:
News release, Greenwich Hospital.
Sabetta, J. Plos ONE, June 16, 2010.
James Sabetta, MD, Yale University School of Medicine, Greenwich Hospital, Greenwich, Conn.
Continue Reading
Simple steps such as eating foods rich with vitamin D and getting more sunshine may help to reduce your chances of contracting flu and other similar illnesses, shows a study by scientists at Yale University School of Medicine and Greenwich Hospital in Connecticut.
"People in the South and West get more sun than in the North, which is good for them, because you get vitamin D from the sun," study researcher James R. Sabetta, MD, of the Yale University School of Medicine and Greenwich Hospital, Conn., tells WebMD. "It's not a panacea, but it helps."
Sabetta and his team of colleagues followed 198 healthy adults during the fall and winter of 2009-2010 to see if declining levels of vitamin D in the fall and winter could be a factor in the seasonal increased prevalence of respiratory viral infections, such as flu.
The study shows people who maintain vitamin D blood levels of 38 nanograms per milliliter or more are less likely to get viral infections such as flu than people with less in their blood.
Of 18 people who maintained that level during the study period, only three developed viral infections.
But of the 180 other participants with less vitamin D in their blood, 81(45%), did get sick with viral infections.
And those with higher levels of vitamin D also experienced a marked reduction in the number of days they were ill, Sabetta tells WebMD.
In addition to getting more sun and consuming milk and foods with vitamin D, he recommends supplements, especially for people in areas with less sunlight and for those who spend daylight hours in darker, indoor environments.
"If you have a level of 38, your risk is down 50%," he tells WebMD. "A lot of people don't have an adequate level, and 38 is a little above what you should have to be considered in the sufficient range. There are a billion people worldwide with levels below 30."
And 30 is considered "sufficient," he says.
Watching for Signs of Illness
Participants in the study had blood samples drawn monthly using a sophisticated technique to accurately measure vitamin D levels. They didn't know that vitamin D was being measured, and even investigators didn't know until the end of the study.
All participants were asked to report signs of illness, such as nasal congestion, sore throat, cough with or without fever, chills, fatigue and general malaise.
Those reporting any symptoms were seen the same day at the study site by one of the infectious disease investigators.
People in the study kept a diary of symptoms and were called every one to three days during the illness to review any signs of symptoms until they were better. The investigators recorded the duration of each symptom, the total duration of the illness, and any antimicrobials administered.
Sabetta says the findings suggest that supplementing vitamin D to achieve a blood level of 38 nanograms per milliliter or higher could result in a significant health benefit by reducing odds of contracting viral infections of the respiratory tract.
But he says more studies are needed to determine the efficacy of vitamin D supplementation in the prevention of infections, including influenza.
The researchers conclude that the lower levels of vitamin D seen during the winter in temperate climates may contribute to the prevalence of influenza in colder months.
The findings, Sabetta says, have significant implications for public health and also may explain the seasonality of certain infections, and also the higher morbidity and mortality of such illnesses in people who are predisposed to lower concentrations of vitamin D.
Sabetta says vitamin D has known effects on the immune system, and the study reinforces the association between vitamin D deficiency and susceptibility to infections of the respiratory tract.
The study is published online in the journal Plos ONE.
Vitamin D levels depend "on how big you are, your skin color, your diet and how much sun exposure you get," Sabetta tells WebMD. "Individuals should get their vitamin D levels checked. If you are gardening a lot, you probably are fine, but people in an office all day may need supplements."
SOURCES:
News release, Greenwich Hospital.
Sabetta, J. Plos ONE, June 16, 2010.
James Sabetta, MD, Yale University School of Medicine, Greenwich Hospital, Greenwich, Conn.
Subscribe to:
Posts (Atom)
H1N1 Can Be Transmitted From Humans to Pets
July 20, 2010 (Atlanta, Georgia) — Cases of H1N1 in cats and ferrets appear to have been transmitted from symptomatic human household members, according to a new analysis from the Oregon Department of Human Services.
Emilio E. DeBess, DVM, from the Oregon Department of Human Services, in Portland, presented the findings here at a poster session at the International Conference on Emerging Infectious Diseases 2010.
"We are reporting the first cluster of laboratory-confirmed cases of H1N1 in the US," the authors note, "in 4 ferrets and 2 cats."
According to Dr. DeBess, the cases represent the first description of the pathology and viral antigen distribution of lethal respiratory disease in domestic cats after natural pandemic (H1N1) 2009 influenza virus infection, which was probably transmitted from humans.
"Pets can be affected by H1N1 by the same means as humans; therefore, patients with H1N1 should also wash their hands and cover their cough to protect their pets," he told Medscape Medical News.
A total of 4 ferrets were infected, presenting with sneezing, coughing, lethargy and nasal discharge. Three of the ferrets had elevated temperatures. In addition, 2 cats affected with H1N1 presented with severe respiratory distress, dyspnea, and cyanosis but did not have an increased temperature. The 2 cats, one a 10-year-old neutered domestic shorthair and the other an 8-year-old spayed domestic shorthair, died shortly after developing severe respiratory disease.
The samples were found to be positive for H1N1 by both the matrix and N1 real-time reverse transcriptase polymer chain reaction assays for the 2009 H1N1 pandemic influenza virus and were subsequently confirmed by the US Department of Agriculture/Animal and Plant Health Inspection Service/Veterinary Services/National Veterinary Services Laboratories.
Marked consolidation of the lung lobes and air bronchograms throughout the chest were observed on x-ray of the cats, and pleural effusion was identified in one. Both cats also exhibited pneumonia and fibrin exudation in bronchioles and alveoli.
According to Dr. DeBess, transmission of H1N1 to pets is the same as it is for humans, but it is unknown at this point whether H1N1 could spread back from pets to humans, although "it could only make sense," he said.
Brett Sponseller, DVM, PhD, assistant professor of vet microbiology and preventive medicine at Iowa State University, in Ames, and colleagues recently reported a similar single case of suspected human-to-cat transmission earlier this year. He commented that the frequency of cross-species transmission to companion animals is unknown at this point. "However, I suspect that it is uncommon, yet underdiagnosed," he told Medscape Medical News. "As in humans, most cases in animals appear to be self-limiting and may go undiagnosed," he added.
According to Dr. Sponseller, with the advent of H1N1, "medical professionals need to be aware of the (reverse) zoonotic potential of this virus and recommend safety precautions to minimize spread to and from companion animals." Recommendations are outlined on the American Veterinary Medical Association Web site.
The authors and commentators have disclosed no relevant financial relationships.
International Conference on Emerging Infectious Diseases (ICEID) 2010: Poster Session. Presented July 13, 2010.
Emilio E. DeBess, DVM, from the Oregon Department of Human Services, in Portland, presented the findings here at a poster session at the International Conference on Emerging Infectious Diseases 2010.
"We are reporting the first cluster of laboratory-confirmed cases of H1N1 in the US," the authors note, "in 4 ferrets and 2 cats."
According to Dr. DeBess, the cases represent the first description of the pathology and viral antigen distribution of lethal respiratory disease in domestic cats after natural pandemic (H1N1) 2009 influenza virus infection, which was probably transmitted from humans.
"Pets can be affected by H1N1 by the same means as humans; therefore, patients with H1N1 should also wash their hands and cover their cough to protect their pets," he told Medscape Medical News.
A total of 4 ferrets were infected, presenting with sneezing, coughing, lethargy and nasal discharge. Three of the ferrets had elevated temperatures. In addition, 2 cats affected with H1N1 presented with severe respiratory distress, dyspnea, and cyanosis but did not have an increased temperature. The 2 cats, one a 10-year-old neutered domestic shorthair and the other an 8-year-old spayed domestic shorthair, died shortly after developing severe respiratory disease.
The samples were found to be positive for H1N1 by both the matrix and N1 real-time reverse transcriptase polymer chain reaction assays for the 2009 H1N1 pandemic influenza virus and were subsequently confirmed by the US Department of Agriculture/Animal and Plant Health Inspection Service/Veterinary Services/National Veterinary Services Laboratories.
Marked consolidation of the lung lobes and air bronchograms throughout the chest were observed on x-ray of the cats, and pleural effusion was identified in one. Both cats also exhibited pneumonia and fibrin exudation in bronchioles and alveoli.
According to Dr. DeBess, transmission of H1N1 to pets is the same as it is for humans, but it is unknown at this point whether H1N1 could spread back from pets to humans, although "it could only make sense," he said.
Brett Sponseller, DVM, PhD, assistant professor of vet microbiology and preventive medicine at Iowa State University, in Ames, and colleagues recently reported a similar single case of suspected human-to-cat transmission earlier this year. He commented that the frequency of cross-species transmission to companion animals is unknown at this point. "However, I suspect that it is uncommon, yet underdiagnosed," he told Medscape Medical News. "As in humans, most cases in animals appear to be self-limiting and may go undiagnosed," he added.
According to Dr. Sponseller, with the advent of H1N1, "medical professionals need to be aware of the (reverse) zoonotic potential of this virus and recommend safety precautions to minimize spread to and from companion animals." Recommendations are outlined on the American Veterinary Medical Association Web site.
The authors and commentators have disclosed no relevant financial relationships.
International Conference on Emerging Infectious Diseases (ICEID) 2010: Poster Session. Presented July 13, 2010.
Antibodies Induced by HIV Vaccines May Give False-Positive HIV Test Results
July 20, 2010 — Trials of vaccines designed to prevent HIV infection might induce antibodies that will cause false-positive results on routine antibody tests for HIV infection. Although the goal of much vaccine development is to induce the production of protective antibodies, they might also cause a state of vaccine-induced seropositivity/reactivity (VISP), which can confound the interpretation of HIV tests in the absence of HIV infection.
In the July 21 issue of JAMA, which focuses on HIV/AIDS, Lindsey Baden, MD, from Brigham and Women's Hospital and Harvard Medical School in Boston, Massachusetts, said that more than 30,000 people have participated in clinical trials of a variety of potential prophylactic vaccines against HIV. These vaccines have used a variety of delivery methods and antigenic compounds and were aimed at inducing different forms of immunity. Dr. Baden discussed the findings at AIDS 2010: XVIII International AIDS Conference in Vienna, Austria.
"The goal of HIV vaccines is to elicit an immune response against HIV. The goal of HIV testing is to see if there is the presence of an immune response to HIV," Dr. Baden explained. "VISP occurs when the antibody or the immune response elicited cross-reacts with the diagnostic test; there can be confusion if one is not aware of this possibility."
VISP might have important ramifications, Dr. Baden said. "Participants may be at risk for misdiagnosis, and if that occurs, then many social harms occur . . . related to insurance, military service, blood [and] tissue donation, immigration, and a variety of other issues that may arise."
Dr. Baden and coworkers studied VISP occurring with various vaccines that were studied by the HIV Vaccine Trials Network. VISP was determined using 3 common US Food and Drug Administration–approved enzyme immunoassay (EIA) test kits. They evaluated VISP in healthy HIV-seronegative adults who participated in any of 25 phase 1 or 2 phase 2a vaccine trials conducted between 2000 and 2010 in the United States and internationally.
VISP was defined as a positive reaction on 1 or more of the EIA tests and a Western blot result that was negative, indeterminate, or atypical-positive — meaning a blot profile consistent with the vaccine product — in conjunction with a negative test for HIV-1 by nucleic acid testing.
Of the 2176 trial participants receiving a test vaccine, 908 (41.7%; 95% confidence interval [CI], 39.6% - 43.8%) had VISP. The occurrence of VISP varied greatly according to the kind of vaccine being tested (e.g., 86.7% for an adenovirus 5 product but only 6.3% for a DNA-alone product). Similarly, results varied substantially according to the test kit used (range, 8.8% to 40.9% VISP).
Dr. Baden concluded that VISP is a common but highly variable outcome in people who participate in vaccine trials. "The occurrence of this is dependent on several factors, including the vaccine or delivery system, the insert used in the vaccine, and the diagnostic test," he said.
These results indicate the need to develop novel rapid-detection methods that do not detect candidate vaccine antigens; several candidate diagnostics are now being investigated that use antigens that are unlikely to be used in vaccines, he noted.
But Dr. Baden said the easiest way to minimize the concern about false-positive test results is for healthcare providers to be aware of the issue as more people participate in vaccine studies. "All they need to do is ask their patients, and if their patients say they are in a vaccine study, then that should be an important consideration in how diagnostic testing is performed," he advised. In addition, testing might best be done by the vaccine trial site, which would be familiar with results related to the specific vaccine.
Because trial participants with VISP might subsequently become infected with HIV, appropriate testing is imperative, Dr. Baden emphasized, including testing for HIV RNA. Trial sites should also test for VISP at the end of the trial and tell participants their VISP status so that they can inform their healthcare providers.
Jason Haukoos, MD, MSc, assistant professor of surgery at the University of Colorado and an emergency physician in the Department of Emergency Medicine at the Denver Health Medical Center, told Medscape Medical News that relatively few patients have been in HIV vaccine trials, so concerns about VISP are small and Dr. Baden's work should go a long way toward solving the problem of false-positive results caused by VISP.
"And there are also a lot of diagnostics coming out now . . . [that will] not only look for antibodies but also antigens," he said. "If you have a combination antibody–antigen assay, then the VISP issue, I think, goes away on some level." He added that, unfortunately, we are still a long way from having an approved vaccine that will be used widely, so the problem of VISP for the near term is minimal.
Melanie Thompson, MD, from the AIDS Research Consortium of Atlanta, Georgia, and chair of the International AIDS Society–USA Antiretroviral Therapy Guidelines Panel, who was not involved in the study, agreed that false-positive HIV test results from VISP are not yet a problem given the small number of trial participants.
"As vaccine studies are expanding, it is going to become a more general issue," she told Medscape Medical News. Patients in vaccine trials will need to be aware of the issue "because they are going to educate the doctors in the community about VISP," she said. "Any HIV testers in the community need to be aware that persons who have been in vaccine trials may have a false-positive HIV test on the antibody testing."
Even in countries with limited healthcare resources, where many of the vaccine trials occur, she said the problem of VISP should be small if the test sites encourage their trial subjects to come back to them for HIV testing, where the proper test methodologies exist, if they have a positive test result elsewhere.
The work was funded by the National Institute of Allergy and Infectious Diseases and by a University of Washington Center for AIDS Research grant. Dr. Baden has disclosed no relevant financial relationships. Dr. Haukoos reports receiving unrestricted research support from Abbott Laboratories and being supported in part by the Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality. Dr. Thompson reports receiving research grants awarded to the AIDS Research Consortium of Atlanta from Abbott Laboratories, Avexa, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, GeoVax, Katketsuken, Koronis Pharmaceuticals, Merck Research Laboratories, Myriad, Ora-Sure, Panacos Pharmaceuticals (now Myriad), Pfizer, Progenics Pharmaceuticals, Roche Laboratories, Roche Molecular Systems, Serono, Theratechnologies Tibotec Therapeutics, Tobira Therapeutics, Trimeris, and VaxGen; serving on the scientific advisory boards or as a clinical trial design consultant for Chimerix, GeoVax, GlaxoSmithKline, Panacos Pharmaceuticals, Progenics Pharmaceuticals, and Tibotec Therapeutics; receiving honoraria for scientific lectures from GlaxoSmithKline and Serono; and serving on data and safety monitoring boards for Tibotec Therapeutics.
JAMA. 2010;304:275-283. Abstract
AIDS 2010: XVIII International AIDS Conference. Presented July 18, 2010.
| |
| Dr. Lindsey Baden |
"The goal of HIV vaccines is to elicit an immune response against HIV. The goal of HIV testing is to see if there is the presence of an immune response to HIV," Dr. Baden explained. "VISP occurs when the antibody or the immune response elicited cross-reacts with the diagnostic test; there can be confusion if one is not aware of this possibility."
VISP might have important ramifications, Dr. Baden said. "Participants may be at risk for misdiagnosis, and if that occurs, then many social harms occur . . . related to insurance, military service, blood [and] tissue donation, immigration, and a variety of other issues that may arise."
Dr. Baden and coworkers studied VISP occurring with various vaccines that were studied by the HIV Vaccine Trials Network. VISP was determined using 3 common US Food and Drug Administration–approved enzyme immunoassay (EIA) test kits. They evaluated VISP in healthy HIV-seronegative adults who participated in any of 25 phase 1 or 2 phase 2a vaccine trials conducted between 2000 and 2010 in the United States and internationally.
VISP was defined as a positive reaction on 1 or more of the EIA tests and a Western blot result that was negative, indeterminate, or atypical-positive — meaning a blot profile consistent with the vaccine product — in conjunction with a negative test for HIV-1 by nucleic acid testing.
Of the 2176 trial participants receiving a test vaccine, 908 (41.7%; 95% confidence interval [CI], 39.6% - 43.8%) had VISP. The occurrence of VISP varied greatly according to the kind of vaccine being tested (e.g., 86.7% for an adenovirus 5 product but only 6.3% for a DNA-alone product). Similarly, results varied substantially according to the test kit used (range, 8.8% to 40.9% VISP).
Dr. Baden concluded that VISP is a common but highly variable outcome in people who participate in vaccine trials. "The occurrence of this is dependent on several factors, including the vaccine or delivery system, the insert used in the vaccine, and the diagnostic test," he said.
These results indicate the need to develop novel rapid-detection methods that do not detect candidate vaccine antigens; several candidate diagnostics are now being investigated that use antigens that are unlikely to be used in vaccines, he noted.
But Dr. Baden said the easiest way to minimize the concern about false-positive test results is for healthcare providers to be aware of the issue as more people participate in vaccine studies. "All they need to do is ask their patients, and if their patients say they are in a vaccine study, then that should be an important consideration in how diagnostic testing is performed," he advised. In addition, testing might best be done by the vaccine trial site, which would be familiar with results related to the specific vaccine.
Because trial participants with VISP might subsequently become infected with HIV, appropriate testing is imperative, Dr. Baden emphasized, including testing for HIV RNA. Trial sites should also test for VISP at the end of the trial and tell participants their VISP status so that they can inform their healthcare providers.
Jason Haukoos, MD, MSc, assistant professor of surgery at the University of Colorado and an emergency physician in the Department of Emergency Medicine at the Denver Health Medical Center, told Medscape Medical News that relatively few patients have been in HIV vaccine trials, so concerns about VISP are small and Dr. Baden's work should go a long way toward solving the problem of false-positive results caused by VISP.
"And there are also a lot of diagnostics coming out now . . . [that will] not only look for antibodies but also antigens," he said. "If you have a combination antibody–antigen assay, then the VISP issue, I think, goes away on some level." He added that, unfortunately, we are still a long way from having an approved vaccine that will be used widely, so the problem of VISP for the near term is minimal.
Melanie Thompson, MD, from the AIDS Research Consortium of Atlanta, Georgia, and chair of the International AIDS Society–USA Antiretroviral Therapy Guidelines Panel, who was not involved in the study, agreed that false-positive HIV test results from VISP are not yet a problem given the small number of trial participants.
"As vaccine studies are expanding, it is going to become a more general issue," she told Medscape Medical News. Patients in vaccine trials will need to be aware of the issue "because they are going to educate the doctors in the community about VISP," she said. "Any HIV testers in the community need to be aware that persons who have been in vaccine trials may have a false-positive HIV test on the antibody testing."
Even in countries with limited healthcare resources, where many of the vaccine trials occur, she said the problem of VISP should be small if the test sites encourage their trial subjects to come back to them for HIV testing, where the proper test methodologies exist, if they have a positive test result elsewhere.
The work was funded by the National Institute of Allergy and Infectious Diseases and by a University of Washington Center for AIDS Research grant. Dr. Baden has disclosed no relevant financial relationships. Dr. Haukoos reports receiving unrestricted research support from Abbott Laboratories and being supported in part by the Centers for Disease Control and Prevention and the Agency for Healthcare Research and Quality. Dr. Thompson reports receiving research grants awarded to the AIDS Research Consortium of Atlanta from Abbott Laboratories, Avexa, Boehringer Ingelheim Pharmaceuticals, Bristol-Myers Squibb, GlaxoSmithKline, Gilead Sciences, GeoVax, Katketsuken, Koronis Pharmaceuticals, Merck Research Laboratories, Myriad, Ora-Sure, Panacos Pharmaceuticals (now Myriad), Pfizer, Progenics Pharmaceuticals, Roche Laboratories, Roche Molecular Systems, Serono, Theratechnologies Tibotec Therapeutics, Tobira Therapeutics, Trimeris, and VaxGen; serving on the scientific advisory boards or as a clinical trial design consultant for Chimerix, GeoVax, GlaxoSmithKline, Panacos Pharmaceuticals, Progenics Pharmaceuticals, and Tibotec Therapeutics; receiving honoraria for scientific lectures from GlaxoSmithKline and Serono; and serving on data and safety monitoring boards for Tibotec Therapeutics.
JAMA. 2010;304:275-283. Abstract
AIDS 2010: XVIII International AIDS Conference. Presented July 18, 2010.
Significant Weight Loss in Obese and Overweight Patients Treated With Lorcaserin
July 15, 2010 (Winter Park, Florida) — Treatment with the investigational obesity drug lorcaserin (Arena Pharmaceuticals, San Diego, CA) results in significantly greater weight loss than placebo in obese or overweight patients, a new study shows [1]. After one year of treatment, patients treated with the novel weight-loss drug lost 4 kg more than those treated with placebo, with significantly more lorcaserin-treated patients losing more than 5% of their body weight compared with the placebo-treated patients.
"I see the obesity epidemic in the US as a medical issue of pretty amazing proportions when you have one-third of the US population that's obese," lead investigator Dr Steven Smith (Florida Hospital, Winter Park) told heartwire . "If you look at the risk for developing diabetes, cardiovascular disease, and all the way down to orthopedic problems, I think there is a huge unmet need out there for multiple medical conditions . . . a huge unmet need that lorcaserin has the potential to fill."
Importantly, an assessment of adverse events at one and two years did not show any difference in the rates of cardiac valvulopathy, a problem that was observed with less selective obesity agents, report investigators. "In phase 3 studies, particularly in obesity studies, the sample size goes up, which gives us a better picture not just of the efficacy, which I don't think requires many thousands of patients, but lets us really nail some of the safety issues regarding lorcaserin," added Smith.
Lorcaserin is a selective serotonin 2C receptor agonist, and previous studies have shown that drugs targeting serotonin are beneficial in weight loss. The nonselective serotonin agonist fenfluramine, which, along with phentermine, made up the antiobesity medication fen-phen, for example, was approved by the Food and Drug Administration (FDA) in the early 1970s as an adjunct for the treatment of obesity. It was pulled from the market after reports it caused valve disease and pulmonary hypertension.
The results of the study, known as the Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) trial, are published in the July 15, 2010 issue of the New England Journal of Medicine.
Safety of the 5-HT2C Receptor
In BLOOM, 3182 obese or overweight patients--defined as a body-mass index (BMI) between 30 to 45 kg/m2 (or BMI 27 to 45 with at least one preexisting condition, including hypertension, diabetes, cardiovascular disease, impaired glucose tolerance, or sleep apnea)--were randomized to lorcaserin 10 mg twice daily or placebo for 52 weeks. All patients were instructed to exercise moderately for 30 minutes daily and to reduce caloric intake 600 kcal below their estimated daily energy requirements.
At one year, 47.5% of the lorcaserin-treated patients lost 5% or more of their body weight compared with 20.3% of the placebo-treated patients. In addition, 22.7% of the lorcaserin-treated individuals lost 10% of their body weight, while just 7.7% lost the same amount of weight in the placebo arm. On average, individuals treated with lorcaserin lost 5.8 kg compared with 2.2 kg lost in the placebo-treated patients.
After one year of treatment, patients who received lorcaserin were randomly assigned 2:1 to continue treatment for an additional year or to receive placebo. The lorcaserin patients switched to placebo regained the weight lost in the first year and ended year two with roughly the same body weight as those who received placebo for the full two-year study.
"I have thought that for not only lorcaserin, but for other pharmacotherapy approaches, that obesity is a chronic condition, like high blood pressure and hypercholesterolemia," said Smith. "We know that, [just like with other drugs], if you stop taking them, just like you saw here, people regain their body weight. I personally believe that long-term treatment with antiobesity therapies matches the disease and makes a lot of sense, just like treating hypertension."
Asked about the 5.8-kg reduction in weight, Smith told heartwire that for individuals with obesity, every pound lost counts. Medically accepted weight loss, he noted, is approximately 5% of body weight and is generally needed to improve cardiovascular risk factors. Overall, there were small but statistically significant improvements in blood pressure, triglycerides, insulin sensitivity, fibrinogen, and C-reactive protein (CRP), among other measures, compared with placebo. "All the arrows are pointing in the right direction," he said.
In BLOOM, the percentage of patients who remained in the trial at one year was 55.4% in the lorcaserin arm and 45.1% in the placebo arm. The high dropout rate, said Smith, is on par with other antiobesity studies and is likely the result of individuals being able to observe the drug effects by stepping on a scale or tightening their belt buckles, and deciding they no longer needed or wanted to take the drug. He noted that the most frequently reported side effects with lorcaserin were headache, dizziness, and nausea, which occurred early and usually went away. That more people stayed on the drug than on placebo speaks to the tolerability of lorcaserin, said Smith.
Regarding the more serious concern of cardiac valvulopathy, the BLOOM investigators report that at one year FDA-defined valvulopathy developed in 2.3% of patients in the placebo arm and 2.7% of patients in the lorcaserin group, a nonsignificant difference. At two years, the rates were similar.
The Heart Valves
Recent evidence suggests that different serotonin receptors are responsible for different effects, with the 5-HT2B receptor believed responsible for the adverse cardiac valvular effects, while the 5-HT2C receptor targeted by lorcaserin is responsible for weight loss. A smaller 12-week study with lorcaserin showed the drug reduced weight without any adverse effects on the cardiac valves or pulmonary arterial pressure. The Behavioral Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) trial, previously reported by heartwire , showed lorcaserin did not increase the risk of cardiac valvulopathy or worsen valve problems, including in some patients with preexisting mild to greater aortic or mitral regurgitation.
Speaking with heartwire , Dr Frank Greenway (Pennington Biomedical Research Center, Baton Rouge, LA), an obesity expert who does research in obesity drug development but was not affiliated with the BLOOM trial, agreed that most of the concerns with antiobesity drugs stemmed from the lack of specificity with earlier agents.
"Everybody was very excited about fen-phen because it caused a 15% to 20% reduction in weight loss, so after it was taken off the market, the pharmaceutical industry tried to developed a drug specific to the 5-HT2C receptor, which is in the brain and regulates appetite," he said. "Judging from the affinity constants and so forth, it would appear there shouldn't be a problem with heart-valve pathology with lorcaserin because it's a specific 5-HT2C receptor agonist."
The lack of valve problems in a study extended to two years suggests this is no longer a problem, and "the issue has been put to rest," said Greenway.
In an editorial accompany the study [2], Dr Arne Astrup (University of Copenhagen, Denmark) writes that the "history of pharmacologic treatment of obesity is characterized by repetition," with drugs approved and then later yanked because of serious adverse effects detected during postmarketing surveillance.
In addition to fenfluramine, Astrup cites rimonabant, which was scrapped by Sanofi-Aventis after concerns were raised about the drug's psychiatric side effects, and sibutramine (Meridia, Abbott Laboratories), which has been removed from the European market and is contraindicated in individuals with a history of cardiovascular disease. Still, despite these setbacks, Astrup notes that "it makes good sense to develop more selective agents" that work on the 5-HT2C receptors.
However, putting lorcaserin on the market to treat obesity should be based not on the greater efficacy of the drug, which is slightly less than other compounds, but on improved safety and an improved adverse-event profile, as well as meaningful benefits on risk factors for type 2 diabetes mellitus and cardiovascular disease. "Given the history, we will need to be doubly sure about the safety of lorcaserin, used either alone or in combination with other weight-loss drugs," writes Astrup.
Arena Pharmaceuticals sponsored the BLOOM study. Smith reports consulting fees/honorarium from Arena Pharmaceuticals and has received financial support for travel expenses related to the BLOOM study. Smith spoke with heartwire on a conference call set up by Russo Partners, a public-relations firm. Present on the call were David Schull (Russo Partners, New York), Lena Evans (Russo Partners), and Cindy McGee (Arena Pharmaceuticals). Astrup reports receiving grant support from NeuroSearch and Novo Nordisk and currently sits on an external advisory board for Merck and NeuroSearch.
"I see the obesity epidemic in the US as a medical issue of pretty amazing proportions when you have one-third of the US population that's obese," lead investigator Dr Steven Smith (Florida Hospital, Winter Park) told heartwire . "If you look at the risk for developing diabetes, cardiovascular disease, and all the way down to orthopedic problems, I think there is a huge unmet need out there for multiple medical conditions . . . a huge unmet need that lorcaserin has the potential to fill."
Importantly, an assessment of adverse events at one and two years did not show any difference in the rates of cardiac valvulopathy, a problem that was observed with less selective obesity agents, report investigators. "In phase 3 studies, particularly in obesity studies, the sample size goes up, which gives us a better picture not just of the efficacy, which I don't think requires many thousands of patients, but lets us really nail some of the safety issues regarding lorcaserin," added Smith.
Lorcaserin is a selective serotonin 2C receptor agonist, and previous studies have shown that drugs targeting serotonin are beneficial in weight loss. The nonselective serotonin agonist fenfluramine, which, along with phentermine, made up the antiobesity medication fen-phen, for example, was approved by the Food and Drug Administration (FDA) in the early 1970s as an adjunct for the treatment of obesity. It was pulled from the market after reports it caused valve disease and pulmonary hypertension.
The results of the study, known as the Behavioral Modification and Lorcaserin for Overweight and Obesity Management (BLOOM) trial, are published in the July 15, 2010 issue of the New England Journal of Medicine.
Safety of the 5-HT2C Receptor
In BLOOM, 3182 obese or overweight patients--defined as a body-mass index (BMI) between 30 to 45 kg/m2 (or BMI 27 to 45 with at least one preexisting condition, including hypertension, diabetes, cardiovascular disease, impaired glucose tolerance, or sleep apnea)--were randomized to lorcaserin 10 mg twice daily or placebo for 52 weeks. All patients were instructed to exercise moderately for 30 minutes daily and to reduce caloric intake 600 kcal below their estimated daily energy requirements.
At one year, 47.5% of the lorcaserin-treated patients lost 5% or more of their body weight compared with 20.3% of the placebo-treated patients. In addition, 22.7% of the lorcaserin-treated individuals lost 10% of their body weight, while just 7.7% lost the same amount of weight in the placebo arm. On average, individuals treated with lorcaserin lost 5.8 kg compared with 2.2 kg lost in the placebo-treated patients.
After one year of treatment, patients who received lorcaserin were randomly assigned 2:1 to continue treatment for an additional year or to receive placebo. The lorcaserin patients switched to placebo regained the weight lost in the first year and ended year two with roughly the same body weight as those who received placebo for the full two-year study.
"I have thought that for not only lorcaserin, but for other pharmacotherapy approaches, that obesity is a chronic condition, like high blood pressure and hypercholesterolemia," said Smith. "We know that, [just like with other drugs], if you stop taking them, just like you saw here, people regain their body weight. I personally believe that long-term treatment with antiobesity therapies matches the disease and makes a lot of sense, just like treating hypertension."
Asked about the 5.8-kg reduction in weight, Smith told heartwire that for individuals with obesity, every pound lost counts. Medically accepted weight loss, he noted, is approximately 5% of body weight and is generally needed to improve cardiovascular risk factors. Overall, there were small but statistically significant improvements in blood pressure, triglycerides, insulin sensitivity, fibrinogen, and C-reactive protein (CRP), among other measures, compared with placebo. "All the arrows are pointing in the right direction," he said.
In BLOOM, the percentage of patients who remained in the trial at one year was 55.4% in the lorcaserin arm and 45.1% in the placebo arm. The high dropout rate, said Smith, is on par with other antiobesity studies and is likely the result of individuals being able to observe the drug effects by stepping on a scale or tightening their belt buckles, and deciding they no longer needed or wanted to take the drug. He noted that the most frequently reported side effects with lorcaserin were headache, dizziness, and nausea, which occurred early and usually went away. That more people stayed on the drug than on placebo speaks to the tolerability of lorcaserin, said Smith.
Regarding the more serious concern of cardiac valvulopathy, the BLOOM investigators report that at one year FDA-defined valvulopathy developed in 2.3% of patients in the placebo arm and 2.7% of patients in the lorcaserin group, a nonsignificant difference. At two years, the rates were similar.
The Heart Valves
Recent evidence suggests that different serotonin receptors are responsible for different effects, with the 5-HT2B receptor believed responsible for the adverse cardiac valvular effects, while the 5-HT2C receptor targeted by lorcaserin is responsible for weight loss. A smaller 12-week study with lorcaserin showed the drug reduced weight without any adverse effects on the cardiac valves or pulmonary arterial pressure. The Behavioral Modification and Lorcaserin Second Study for Obesity Management (BLOSSOM) trial, previously reported by heartwire , showed lorcaserin did not increase the risk of cardiac valvulopathy or worsen valve problems, including in some patients with preexisting mild to greater aortic or mitral regurgitation.
Speaking with heartwire , Dr Frank Greenway (Pennington Biomedical Research Center, Baton Rouge, LA), an obesity expert who does research in obesity drug development but was not affiliated with the BLOOM trial, agreed that most of the concerns with antiobesity drugs stemmed from the lack of specificity with earlier agents.
"Everybody was very excited about fen-phen because it caused a 15% to 20% reduction in weight loss, so after it was taken off the market, the pharmaceutical industry tried to developed a drug specific to the 5-HT2C receptor, which is in the brain and regulates appetite," he said. "Judging from the affinity constants and so forth, it would appear there shouldn't be a problem with heart-valve pathology with lorcaserin because it's a specific 5-HT2C receptor agonist."
The lack of valve problems in a study extended to two years suggests this is no longer a problem, and "the issue has been put to rest," said Greenway.
In an editorial accompany the study [2], Dr Arne Astrup (University of Copenhagen, Denmark) writes that the "history of pharmacologic treatment of obesity is characterized by repetition," with drugs approved and then later yanked because of serious adverse effects detected during postmarketing surveillance.
In addition to fenfluramine, Astrup cites rimonabant, which was scrapped by Sanofi-Aventis after concerns were raised about the drug's psychiatric side effects, and sibutramine (Meridia, Abbott Laboratories), which has been removed from the European market and is contraindicated in individuals with a history of cardiovascular disease. Still, despite these setbacks, Astrup notes that "it makes good sense to develop more selective agents" that work on the 5-HT2C receptors.
However, putting lorcaserin on the market to treat obesity should be based not on the greater efficacy of the drug, which is slightly less than other compounds, but on improved safety and an improved adverse-event profile, as well as meaningful benefits on risk factors for type 2 diabetes mellitus and cardiovascular disease. "Given the history, we will need to be doubly sure about the safety of lorcaserin, used either alone or in combination with other weight-loss drugs," writes Astrup.
Arena Pharmaceuticals sponsored the BLOOM study. Smith reports consulting fees/honorarium from Arena Pharmaceuticals and has received financial support for travel expenses related to the BLOOM study. Smith spoke with heartwire on a conference call set up by Russo Partners, a public-relations firm. Present on the call were David Schull (Russo Partners, New York), Lena Evans (Russo Partners), and Cindy McGee (Arena Pharmaceuticals). Astrup reports receiving grant support from NeuroSearch and Novo Nordisk and currently sits on an external advisory board for Merck and NeuroSearch.
New AAN Guideline Advocates MRI Over CT for Stroke Diagnosis
July 15, 2010 — After reviewing the relevant literature, a panel of neurologists, neuroradiologists, and radiologists has concluded that diffusion-weighted imaging (DWI) MRI is superior to noncontrast computed tomography (CT) scans, which are the current imaging standard, for diagnosing acute ischemic stroke within 12 hours of symptom onset.
However, although the research shows that DWI is better than CT, the decision about which imaging test to use in clinical practice will depend on issues such as availability and cost, the panel concludes in the new guideline from the American Academy of Neurology.
"The doctors taking care of acute stroke patients, as well as the patients themselves, need to be aware that MRI-DWI is a superior diagnostic tool in acute stroke less than 12 hours," lead author Peter Schellinger, MD, PhD, from Johannes Wesling Clinical Center and the University of Erlangen, Germany, told Medscape Medical News.
"Whether this translates into a change in practice remains to be seen," Dr. Schellinger added. "Logistical, financial, and personnel requirements need to be weighed against better diagnosis which — and this was not within the scope of our assessment — may influence management and ultimately the outcome of the stroke."
The panel's recommendations appear in the July 13 issue of Neurology.
For their review, the panel members addressed 2 questions:
The panel classified evidence using a 4-tier classification scheme for diagnostic (question 1) and prognostic (question 2) articles. Recommendations were based on these levels of evidence.
For question 1, the panel identified 62 articles that fulfilled the inclusion criteria. For DWI, there was 1 class I and 3 class II studies. All PWI studies were class IV.
The class I study assessed the accuracy of MRI (DWI and gradient echo scans) vs CT in 356 consecutive patients presenting to a hospital emergency department over 18 months with a possible diagnosis of acute stroke. In the subset of 221 patients scanned within 12 hours of symptom onset, the majority of blinded readers correctly diagnosed acute ischemic stroke by MRI more often than by CT (94 vs 22; P < .0001).
Single Study Justifies Level A Recommendation
"The odds ratio and its 95% confidence interval (CI) of the difference in the proportions was 25 (8-79), indicating an effect size sufficiently large for this single study to justify a Level A recommendation," the authors write. "A similar direction and magnitude of difference were also seen in the subset of 90 patients scanned within 3 hours of onset."
They added that the sensitivity, specificity, and accuracy of DWI in this study were 77%, 96%, and 86% respectively, compared with 16%, 97% and 55% for CT.
One of the class II studies prospectively evaluated 50 patients with ischemic stroke and 4 with transient ischemic attacks. Patients were randomly assigned to receive MRI or CT within 6 hours of stroke onset. The sensitivity of infarct detection by experts blinded to the patients' symptoms but aware that it was an ischemic stroke population was significantly better with DWI (91%) than CT (61%), as was the accuracy (DWI, 91%; CT, 61%).
The sensitivity of DWI for the diagnosis of ischemic stroke in patients with possible stroke is not perfect; the panel found that its "true sensitivity" is probably 80% to 90%.
The panel concluded from this and the other evidence that DWI is superior to CT for the diagnosis of acute ischemic stroke within 12 hours of symptom onset.
For question 2, the panel identified 8 studies fulfilling the inclusion criteria — 4 class IV studies that assessed only the correlation of baseline DWI and PWI lesion volume with chronic lesion volume, 1 class II study that assessed only the correlation of baseline DWI and PWI lesion volume with clinical outcome, and 2 class II studies and 1 class III study that assessed both clinical and morphologic outcomes. None of the studies compared CT with MRI in predicting these outcomes.
From this research, the panel concluded that baseline DWI volume probably predicts baseline clinical stroke severity and final lesion volume in anterior-circulation stroke syndromes and is possibly accurate in predicting clinical outcome. In addition, the evidence showed that baseline DWI volume possibly does not predict the baseline National Institute of Health Stroke Scale score in posterior circulation stroke syndromes.
In addition to its recommendation that DWI should be considered superior to CT for the diagnosis of ischemic stroke in patients presenting within 12 hours of symptom onset, the panel recommended that baseline DWI volume should be considered useful in predicting baseline clinical stroke severity and final lesion volume in anterior-circulation stroke syndromes, but not in predicting baseline National Institute of Health Stroke Scale score in posterior-circulation stroke syndromes.
The panel found there was insufficient evidence to support or refute the value of PWI in diagnosing acute ischemic stroke but said baseline PWI volume may be considered useful in predicting baseline clinical stroke severity. Prospective, well-designed studies are needed to investigate the diagnostic utility of PWI in acute stroke, according to the panel.
When CT Is the Best Diagnostic Tool
Still, there are circumstances under which CT should remain the primary diagnostic tool, said Dr. Schellinger. "Most typically, this would be in comatose patients, patients with contraindications for MRI such as implanted cardiac pacemakers, and in patients who are candidates for intravenous thrombolytic therapy with rt-PA [tissue plasminogen activator]. Here, a CT-based exclusion of intracranial hemorrhage is enough to make a treatment decision."
A plain CT scan is usually performed faster than a multisequence MRI scan, noted Dr. Schellinger. "Loss of time from arrival at the hospital to initiation of thrombolytic therapy is associated with a loss of efficacy and reduction of chance for a good outcome, and potentially also with a higher bleeding risk, and therefore should be avoided by all means."
In situations in which CT was performed first — for example, in a candidate for thrombolysis — and diagnostic uncertainty remains, MRI may be performed in addition to CT after initiation of thrombolytic therapy to optimize diagnostic assessment, added Dr. Schellinger.
A disadvantage of MRI imaging in acute stroke is its relatively high cost. According to Dr. Schellinger, superior technologists often cost more, although this study did not address cost implications of using MRI to diagnose stroke. "Our objective was an assessment; how and whether this is taken as a means to change [emergency department] practice and stroke care practice remains to be seen."
Lack of Availability
Another perceived disadvantage of MRI is its lack of ready availability. MRIs should be as accessible as CT scans in typical hospital settings, said Dr. Schellinger, adding that the technology has been available at all hours in every center he himself has worked in. "Many of the major stroke services in the United States have implemented MRI as an emergency imaging tool. It is a question of dedication and also a question of whether stroke patients should be treated in stroke centers or not."
Until now, noncontrast CT has been the diagnostic standard for acute stroke. "There was nothing else available, and it was clear pretty early that the most important differential diagnosis of acute ischemic stroke — for example, intracranial hemorrhage — can be detected by CT with a close to 100% sensitivity," explained Dr. Schellinger. "By deduction, it is assumed that a clear stroke syndrome that is not caused by hemorrhage likely is caused by ischemic stroke, even if the CT does not show it."
Best Identification of Early Strokes
Approached for a comment, Gary Abrams, MD, professor of neurology at the University of California–San Francisco, said the new guideline is "very timely and important," as therapeutic interventions for acute ischemic stroke continue to advance.
The article validates what most clinicians already know — that DWI is the most effective and sensitive way to diagnose an acute stroke and is superior to CT — said Dr. Abrams. "As we move forward in the future, this may the way that we need to go in terms of best identification of early strokes and identifying the group that's most amenable to treatment."
As well, the guideline begins to address the issue of PWI, added Dr. Abrams. "This is much less widespread in terms of availability and clinical impact, but it's important, and as the authors suggest, the combination of DWI and PWI may turn out to be the most effective way to understand what's going on in terms of diagnosis and prognosis in acute stroke."
Dr. Schellinger has served or serves on scientific advisory boards for Boehringer Ingelheim, ImaRx Therapeutics, Photothera, Cerevast, and CoAxia Inc; has served or serves on speakers' bureaus for and received funding for travel and speaker honoraria from Boehringer Ingelheim, Sanofi, ImaRx Therapeutics, Photothera, Cerevast, CoAxia Inc, Solvay Pharmaceuticals Inc, and GlaxoSmithKline; serves on editorial boards of Stroke and European Neurology; receives royalties from the publication of NeuroIntensiv (Springer, 2008) and received royalties from the publication of Stroke MRI (Steinkopff, 2004); has served as a consultant for CoAxia Inc, Photothera, Cerevast, ImaRx, and Boehringer Ingelheim; and has provided expert testimony, affidavits, and acted as a witness or consultant in legal proceedings. For disclosure information on the other authors, please see the original article.
Neurology. 2010;75:177-185.
However, although the research shows that DWI is better than CT, the decision about which imaging test to use in clinical practice will depend on issues such as availability and cost, the panel concludes in the new guideline from the American Academy of Neurology.
"The doctors taking care of acute stroke patients, as well as the patients themselves, need to be aware that MRI-DWI is a superior diagnostic tool in acute stroke less than 12 hours," lead author Peter Schellinger, MD, PhD, from Johannes Wesling Clinical Center and the University of Erlangen, Germany, told Medscape Medical News.
"Whether this translates into a change in practice remains to be seen," Dr. Schellinger added. "Logistical, financial, and personnel requirements need to be weighed against better diagnosis which — and this was not within the scope of our assessment — may influence management and ultimately the outcome of the stroke."
The panel's recommendations appear in the July 13 issue of Neurology.
For their review, the panel members addressed 2 questions:
- Are DWI and PWI (perfusion-weighted imaging) sensitive and specific in the diagnosis of acute ischemic stroke compared with concurrent imaging with other techniques, established by follow-up imaging, clinical follow-up, and final discharge diagnosis?
- Does the volume of the DWI or PWI abnormality predict initial clinical severity, final infarct size, and late clinical outcome?
The panel classified evidence using a 4-tier classification scheme for diagnostic (question 1) and prognostic (question 2) articles. Recommendations were based on these levels of evidence.
For question 1, the panel identified 62 articles that fulfilled the inclusion criteria. For DWI, there was 1 class I and 3 class II studies. All PWI studies were class IV.
The class I study assessed the accuracy of MRI (DWI and gradient echo scans) vs CT in 356 consecutive patients presenting to a hospital emergency department over 18 months with a possible diagnosis of acute stroke. In the subset of 221 patients scanned within 12 hours of symptom onset, the majority of blinded readers correctly diagnosed acute ischemic stroke by MRI more often than by CT (94 vs 22; P < .0001).
Single Study Justifies Level A Recommendation
"The odds ratio and its 95% confidence interval (CI) of the difference in the proportions was 25 (8-79), indicating an effect size sufficiently large for this single study to justify a Level A recommendation," the authors write. "A similar direction and magnitude of difference were also seen in the subset of 90 patients scanned within 3 hours of onset."
They added that the sensitivity, specificity, and accuracy of DWI in this study were 77%, 96%, and 86% respectively, compared with 16%, 97% and 55% for CT.
One of the class II studies prospectively evaluated 50 patients with ischemic stroke and 4 with transient ischemic attacks. Patients were randomly assigned to receive MRI or CT within 6 hours of stroke onset. The sensitivity of infarct detection by experts blinded to the patients' symptoms but aware that it was an ischemic stroke population was significantly better with DWI (91%) than CT (61%), as was the accuracy (DWI, 91%; CT, 61%).
The sensitivity of DWI for the diagnosis of ischemic stroke in patients with possible stroke is not perfect; the panel found that its "true sensitivity" is probably 80% to 90%.
The panel concluded from this and the other evidence that DWI is superior to CT for the diagnosis of acute ischemic stroke within 12 hours of symptom onset.
For question 2, the panel identified 8 studies fulfilling the inclusion criteria — 4 class IV studies that assessed only the correlation of baseline DWI and PWI lesion volume with chronic lesion volume, 1 class II study that assessed only the correlation of baseline DWI and PWI lesion volume with clinical outcome, and 2 class II studies and 1 class III study that assessed both clinical and morphologic outcomes. None of the studies compared CT with MRI in predicting these outcomes.
From this research, the panel concluded that baseline DWI volume probably predicts baseline clinical stroke severity and final lesion volume in anterior-circulation stroke syndromes and is possibly accurate in predicting clinical outcome. In addition, the evidence showed that baseline DWI volume possibly does not predict the baseline National Institute of Health Stroke Scale score in posterior circulation stroke syndromes.
In addition to its recommendation that DWI should be considered superior to CT for the diagnosis of ischemic stroke in patients presenting within 12 hours of symptom onset, the panel recommended that baseline DWI volume should be considered useful in predicting baseline clinical stroke severity and final lesion volume in anterior-circulation stroke syndromes, but not in predicting baseline National Institute of Health Stroke Scale score in posterior-circulation stroke syndromes.
The panel found there was insufficient evidence to support or refute the value of PWI in diagnosing acute ischemic stroke but said baseline PWI volume may be considered useful in predicting baseline clinical stroke severity. Prospective, well-designed studies are needed to investigate the diagnostic utility of PWI in acute stroke, according to the panel.
When CT Is the Best Diagnostic Tool
Still, there are circumstances under which CT should remain the primary diagnostic tool, said Dr. Schellinger. "Most typically, this would be in comatose patients, patients with contraindications for MRI such as implanted cardiac pacemakers, and in patients who are candidates for intravenous thrombolytic therapy with rt-PA [tissue plasminogen activator]. Here, a CT-based exclusion of intracranial hemorrhage is enough to make a treatment decision."
A plain CT scan is usually performed faster than a multisequence MRI scan, noted Dr. Schellinger. "Loss of time from arrival at the hospital to initiation of thrombolytic therapy is associated with a loss of efficacy and reduction of chance for a good outcome, and potentially also with a higher bleeding risk, and therefore should be avoided by all means."
In situations in which CT was performed first — for example, in a candidate for thrombolysis — and diagnostic uncertainty remains, MRI may be performed in addition to CT after initiation of thrombolytic therapy to optimize diagnostic assessment, added Dr. Schellinger.
A disadvantage of MRI imaging in acute stroke is its relatively high cost. According to Dr. Schellinger, superior technologists often cost more, although this study did not address cost implications of using MRI to diagnose stroke. "Our objective was an assessment; how and whether this is taken as a means to change [emergency department] practice and stroke care practice remains to be seen."
Lack of Availability
Another perceived disadvantage of MRI is its lack of ready availability. MRIs should be as accessible as CT scans in typical hospital settings, said Dr. Schellinger, adding that the technology has been available at all hours in every center he himself has worked in. "Many of the major stroke services in the United States have implemented MRI as an emergency imaging tool. It is a question of dedication and also a question of whether stroke patients should be treated in stroke centers or not."
Until now, noncontrast CT has been the diagnostic standard for acute stroke. "There was nothing else available, and it was clear pretty early that the most important differential diagnosis of acute ischemic stroke — for example, intracranial hemorrhage — can be detected by CT with a close to 100% sensitivity," explained Dr. Schellinger. "By deduction, it is assumed that a clear stroke syndrome that is not caused by hemorrhage likely is caused by ischemic stroke, even if the CT does not show it."
Best Identification of Early Strokes
Approached for a comment, Gary Abrams, MD, professor of neurology at the University of California–San Francisco, said the new guideline is "very timely and important," as therapeutic interventions for acute ischemic stroke continue to advance.
The article validates what most clinicians already know — that DWI is the most effective and sensitive way to diagnose an acute stroke and is superior to CT — said Dr. Abrams. "As we move forward in the future, this may the way that we need to go in terms of best identification of early strokes and identifying the group that's most amenable to treatment."
As well, the guideline begins to address the issue of PWI, added Dr. Abrams. "This is much less widespread in terms of availability and clinical impact, but it's important, and as the authors suggest, the combination of DWI and PWI may turn out to be the most effective way to understand what's going on in terms of diagnosis and prognosis in acute stroke."
Dr. Schellinger has served or serves on scientific advisory boards for Boehringer Ingelheim, ImaRx Therapeutics, Photothera, Cerevast, and CoAxia Inc; has served or serves on speakers' bureaus for and received funding for travel and speaker honoraria from Boehringer Ingelheim, Sanofi, ImaRx Therapeutics, Photothera, Cerevast, CoAxia Inc, Solvay Pharmaceuticals Inc, and GlaxoSmithKline; serves on editorial boards of Stroke and European Neurology; receives royalties from the publication of NeuroIntensiv (Springer, 2008) and received royalties from the publication of Stroke MRI (Steinkopff, 2004); has served as a consultant for CoAxia Inc, Photothera, Cerevast, ImaRx, and Boehringer Ingelheim; and has provided expert testimony, affidavits, and acted as a witness or consultant in legal proceedings. For disclosure information on the other authors, please see the original article.
Neurology. 2010;75:177-185.
Fish Oil May Lower Risk for Breast Cancer in Postmenopausal Women
July 12, 2010 — Fish oil supplement intake is associated with a lower risk for breast cancer in postmenopausal women, according to the results of the VITamins And Lifestyle (VITAL) Cohort study reported in the July issue of Cancer Epidemiology, Biomarkers & Prevention.
"Use of nonvitamin, nonmineral 'specialty' supplements has increased substantially over recent decades," write Theodore M. Brasky, from Hutchinson Cancer Research Center, University of Washington in Seattle, and colleagues. "Several supplements may have anti-inflammatory or anticancer properties. Additionally, supplements taken for symptoms of menopause have been associated with reduced risk of breast cancer in two case-control studies [but] there have been no prospective studies of the association between the long-term use of these supplements and breast cancer risk."
At baseline in 2000 to 2002, a total of 35,016 postmenopausal women, aged 50 to 76 years and living in western Washington State, completed a 24-page questionnaire concerning their use of specialty supplements. Use was characterized by recency (current vs past), frequency (days/week), and duration (number of years). The Surveillance, Epidemiology, and End Results (SEER) registry showed that from 2000 to 2007, there were 880 incident invasive breast cancers. Cox proportional hazards models allowed estimation of multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs).
For ductal, but not lobular, cancers, current use of fish oil was associated with a reduced risk for breast cancer (HR, 0.68; 95% CI, 0.50 - 0.92), and 10-year average use suggested a trend toward a lower risk (P = .09). Use of the other specialty supplements, including those sometimes taken for menopausal symptoms (black cohosh, dong quai, soy, or St. John's wort) was not associated with breast cancer risk.
"Fish oil may be inversely associated with breast cancer risk," the study authors write. "Fish oil is a potential candidate for chemoprevention studies. Until that time, it is not recommended for individual use for breast cancer prevention."
Limitations of this study include lack of data on supplement dose, reliance on self-report, lack of updated exposure information after baseline, power limited by the relatively low prevalence of use of some specialty supplements, and the possibility of chance findings because 15 specialty supplements were examined.
"[T]his is the first prospective study to report on the association of specialty supplements with breast cancer risk," the study authors conclude. "Our finding of a reduced risk of breast cancer with use of fish oil warrants further study of this agent, focused particularly on timing of exposure and dose, as well as on mechanisms of action that might explain differences by tumor stage or histologic type."
The National Institutes of Health, National Cancer Institute supported this study. The study authors have disclosed no relevant financial relationships.
Cancer Epidemiol Biomarkers Prev. 2010;19:1696-1708. Abstract
"Use of nonvitamin, nonmineral 'specialty' supplements has increased substantially over recent decades," write Theodore M. Brasky, from Hutchinson Cancer Research Center, University of Washington in Seattle, and colleagues. "Several supplements may have anti-inflammatory or anticancer properties. Additionally, supplements taken for symptoms of menopause have been associated with reduced risk of breast cancer in two case-control studies [but] there have been no prospective studies of the association between the long-term use of these supplements and breast cancer risk."
At baseline in 2000 to 2002, a total of 35,016 postmenopausal women, aged 50 to 76 years and living in western Washington State, completed a 24-page questionnaire concerning their use of specialty supplements. Use was characterized by recency (current vs past), frequency (days/week), and duration (number of years). The Surveillance, Epidemiology, and End Results (SEER) registry showed that from 2000 to 2007, there were 880 incident invasive breast cancers. Cox proportional hazards models allowed estimation of multivariable-adjusted hazard ratios (HRs) and 95% confidence intervals (CIs).
For ductal, but not lobular, cancers, current use of fish oil was associated with a reduced risk for breast cancer (HR, 0.68; 95% CI, 0.50 - 0.92), and 10-year average use suggested a trend toward a lower risk (P = .09). Use of the other specialty supplements, including those sometimes taken for menopausal symptoms (black cohosh, dong quai, soy, or St. John's wort) was not associated with breast cancer risk.
"Fish oil may be inversely associated with breast cancer risk," the study authors write. "Fish oil is a potential candidate for chemoprevention studies. Until that time, it is not recommended for individual use for breast cancer prevention."
Limitations of this study include lack of data on supplement dose, reliance on self-report, lack of updated exposure information after baseline, power limited by the relatively low prevalence of use of some specialty supplements, and the possibility of chance findings because 15 specialty supplements were examined.
"[T]his is the first prospective study to report on the association of specialty supplements with breast cancer risk," the study authors conclude. "Our finding of a reduced risk of breast cancer with use of fish oil warrants further study of this agent, focused particularly on timing of exposure and dose, as well as on mechanisms of action that might explain differences by tumor stage or histologic type."
The National Institutes of Health, National Cancer Institute supported this study. The study authors have disclosed no relevant financial relationships.
Cancer Epidemiol Biomarkers Prev. 2010;19:1696-1708. Abstract
Childhood Adversities May Be Risk Factors for Suicidal Behavior Throughout Life
July 2, 2010 — Children who experience serious adversities are at a greater risk for the onset and persistence of suicidal behavior throughout life, according to a new multicountry survey study.
Although exposure to many different adversities "are powerful predictors" for suicidal behavior in later life, sexual or physical abuse during childhood are the strongest risk factors of all, write lead study author Ronny Bruffaerts, PhD, associate professor of psychiatry in the Department of Neurosciences at Katholieke Universiteit Leuven in Belgium, and colleagues.
"Even after rigorously controlling for a broad set of variables, there was at least a threefold increase in lifetime suicide attempt and lifetime suicide ideation among individuals with a history of sexual or physical abuse," they note.
"The point is that childhood trauma has a systematic strong predictive value towards both worse mental and somatic health, as well as increased suicidal behavior," Dr. Bruffaerts told Medscape Medical News.
"Identifying those families at risk of problems, and offering help, may be a way of decreasing suicide around the world," he added in a statement.
The study is published in the July issue of the British Journal of Psychiatry.
Worldwide Suicide Rate Increasing
"Suicides are still one of the major causes of death worldwide," said Dr. Bruffaerts. He added that a report from the World Health Organization showed that the worldwide suicide rate has been increasing steadily since the 1950s.
"Most research on predictors of suicidal behavior focuses on mental disorders as main risk factors, but in the past years there have been some important findings that linked adversities with suicidality," he noted. Also, "the field of childhood adversities is especially an important field to study because its long-term effects have not been studied extensively."
For this study, the investigators evaluated data from nationally representative samples from the World Mental Health surveys. A total of 55,299 people from 21 countries in Africa, the Americas, Asia and the Pacific, Europe, and the Middle East were included.
All participants were interviewed in person about their childhood and whether they had experienced any of the following before they turned 18 years of age: physical abuse, sexual abuse, neglect, parental death, parent divorce, other parental loss, family violence, physical illness, and financial adversity.
Core diagnostic assessments of mental disorders were also made at that time, and the Composite International Diagnostic Interview 3.0 was used to assess lifetime suicidal behavior.
By using these surveys, "we were able to check whether this association [between adversities and suicidal behaviors] held for different countries, different cultures, and different contexts," said Dr. Bruffaerts.
Strong Associations Found
Results showed that 12.2% of the study participants had experienced the death of a parent, 8% had been the victim of physical abuse, and 6.9% had experienced family violence.
A total of 2.7% reported at least 1 suicide attempt, and 9.4% said they had thought about killing themselves.
Among those who had tried to kill themselves, 29.3% had been the victim of physical abuse, 24.8% had experienced family violence, and 14.5% had been sexually abused.
In both bivariate and multivariate models, the childhood adversities were associated with an increased risk for suicide attempt and ideation (odd ratio [OR] range, 1.2 – 5.7) and "the risk increased with the number of adversities experienced, but at a decreasing rate," report the study authors.
In the bivariate models, physical and sexual abuse had the highest odds for suicide attempts (OR, 3.7 and 5.7, respectively) and for suicide ideation (OR, 2.7 and 3.4, respectively). In multivariate additive models, "odds ratios decreased but none lost their statistical significance," the study authors write.
In addition, "associations remained similar after additional adjustment for respondents' lifetime mental disorder status," they add.
Finally, significantly strong associations were found between childhood adversities and suicide attempts in childhood (median OR, 3.8). These associations decreased during the teen years (median OR, 2.5) and in young adulthood (median OR, 2.0) before increasing again in later adulthood (median OR, 2.3).
"Specifically, a history of childhood sexual abuse was associated with a 10.9-fold increase in the odds of a [suicide] attempt between the ages of 4 and 12 years, a 6.1-fold increase in the odds of an attempt between the ages of 13 and 19 years, and a 2.9-fold increase among those between the ages of 20 and 29 years," write the study authors.
Associations Valid Worldwide
The study's overall finding of a strong association between childhood adversities and suicidal behaviors "was not really surprising because prior studies have also shown this," said Dr. Bruffaerts.
However, he noted that "important new findings" included the lifelong effects of childhood adversities, that their highest impact was in childhood and teen years, and that "bodily intrusive adversities" had a stronger impact than other events. But again, "this was not really surprising because it fits with impressions clinicians have."
"That we were able to show that the associations are valid for general populations worldwide was a major step ahead," added Dr. Bruffaerts. "I was [also] surprised...how prevalent childhood adversities actually are in the general population."
When asked about his plans for future research, Dr. Bruffaerts said that a valuable next step pertains to the question, "How can we change the suicidal process?"
"We know that the suicidal process is a treatable condition, but we don’t know exactly how many suicidal persons actually receive treatment," he explained. "In general, I think it is quintessential to generate prevalence estimates of mental disorders worldwide, their correlates and predictors, [and] the ways and why people with psychological problems seek help or not.
"In my opinion, this is an important way to gather data to build national and country-specific policies on," concluded Dr. Bruffaerts.
This study was funded by the US National Institute of Mental Health, the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the US Public Health Service, the Fogarty International Center, the Pan American Health Organization, the Eli Lilly & Company Foundation, Ortho-McNeil Pharmaceutical, GlaxoSmithKline, and Bristol-Myers Squibb. A list of all funders for the various countries' individual health surveys can be found in the original article. Dr. Bruffaerts and all but one of the study authors have disclosed no relevant financial relationships. Investigational team member Ronald C. Kessler reported several declarations of interest, which are listed in full in the original article.
Br J Psychiatry. 2010;197:20-27.
Although exposure to many different adversities "are powerful predictors" for suicidal behavior in later life, sexual or physical abuse during childhood are the strongest risk factors of all, write lead study author Ronny Bruffaerts, PhD, associate professor of psychiatry in the Department of Neurosciences at Katholieke Universiteit Leuven in Belgium, and colleagues.
"Even after rigorously controlling for a broad set of variables, there was at least a threefold increase in lifetime suicide attempt and lifetime suicide ideation among individuals with a history of sexual or physical abuse," they note.
"The point is that childhood trauma has a systematic strong predictive value towards both worse mental and somatic health, as well as increased suicidal behavior," Dr. Bruffaerts told Medscape Medical News.
"Identifying those families at risk of problems, and offering help, may be a way of decreasing suicide around the world," he added in a statement.
The study is published in the July issue of the British Journal of Psychiatry.
Worldwide Suicide Rate Increasing
"Suicides are still one of the major causes of death worldwide," said Dr. Bruffaerts. He added that a report from the World Health Organization showed that the worldwide suicide rate has been increasing steadily since the 1950s.
"Most research on predictors of suicidal behavior focuses on mental disorders as main risk factors, but in the past years there have been some important findings that linked adversities with suicidality," he noted. Also, "the field of childhood adversities is especially an important field to study because its long-term effects have not been studied extensively."
For this study, the investigators evaluated data from nationally representative samples from the World Mental Health surveys. A total of 55,299 people from 21 countries in Africa, the Americas, Asia and the Pacific, Europe, and the Middle East were included.
All participants were interviewed in person about their childhood and whether they had experienced any of the following before they turned 18 years of age: physical abuse, sexual abuse, neglect, parental death, parent divorce, other parental loss, family violence, physical illness, and financial adversity.
Core diagnostic assessments of mental disorders were also made at that time, and the Composite International Diagnostic Interview 3.0 was used to assess lifetime suicidal behavior.
By using these surveys, "we were able to check whether this association [between adversities and suicidal behaviors] held for different countries, different cultures, and different contexts," said Dr. Bruffaerts.
Strong Associations Found
Results showed that 12.2% of the study participants had experienced the death of a parent, 8% had been the victim of physical abuse, and 6.9% had experienced family violence.
A total of 2.7% reported at least 1 suicide attempt, and 9.4% said they had thought about killing themselves.
Among those who had tried to kill themselves, 29.3% had been the victim of physical abuse, 24.8% had experienced family violence, and 14.5% had been sexually abused.
In both bivariate and multivariate models, the childhood adversities were associated with an increased risk for suicide attempt and ideation (odd ratio [OR] range, 1.2 – 5.7) and "the risk increased with the number of adversities experienced, but at a decreasing rate," report the study authors.
In the bivariate models, physical and sexual abuse had the highest odds for suicide attempts (OR, 3.7 and 5.7, respectively) and for suicide ideation (OR, 2.7 and 3.4, respectively). In multivariate additive models, "odds ratios decreased but none lost their statistical significance," the study authors write.
In addition, "associations remained similar after additional adjustment for respondents' lifetime mental disorder status," they add.
Finally, significantly strong associations were found between childhood adversities and suicide attempts in childhood (median OR, 3.8). These associations decreased during the teen years (median OR, 2.5) and in young adulthood (median OR, 2.0) before increasing again in later adulthood (median OR, 2.3).
"Specifically, a history of childhood sexual abuse was associated with a 10.9-fold increase in the odds of a [suicide] attempt between the ages of 4 and 12 years, a 6.1-fold increase in the odds of an attempt between the ages of 13 and 19 years, and a 2.9-fold increase among those between the ages of 20 and 29 years," write the study authors.
Associations Valid Worldwide
The study's overall finding of a strong association between childhood adversities and suicidal behaviors "was not really surprising because prior studies have also shown this," said Dr. Bruffaerts.
However, he noted that "important new findings" included the lifelong effects of childhood adversities, that their highest impact was in childhood and teen years, and that "bodily intrusive adversities" had a stronger impact than other events. But again, "this was not really surprising because it fits with impressions clinicians have."
"That we were able to show that the associations are valid for general populations worldwide was a major step ahead," added Dr. Bruffaerts. "I was [also] surprised...how prevalent childhood adversities actually are in the general population."
When asked about his plans for future research, Dr. Bruffaerts said that a valuable next step pertains to the question, "How can we change the suicidal process?"
"We know that the suicidal process is a treatable condition, but we don’t know exactly how many suicidal persons actually receive treatment," he explained. "In general, I think it is quintessential to generate prevalence estimates of mental disorders worldwide, their correlates and predictors, [and] the ways and why people with psychological problems seek help or not.
"In my opinion, this is an important way to gather data to build national and country-specific policies on," concluded Dr. Bruffaerts.
This study was funded by the US National Institute of Mental Health, the John D. and Catherine T. MacArthur Foundation, the Pfizer Foundation, the US Public Health Service, the Fogarty International Center, the Pan American Health Organization, the Eli Lilly & Company Foundation, Ortho-McNeil Pharmaceutical, GlaxoSmithKline, and Bristol-Myers Squibb. A list of all funders for the various countries' individual health surveys can be found in the original article. Dr. Bruffaerts and all but one of the study authors have disclosed no relevant financial relationships. Investigational team member Ronald C. Kessler reported several declarations of interest, which are listed in full in the original article.
Br J Psychiatry. 2010;197:20-27.
New Guidelines Emphasize the Need for Cancer Patients to Exercise
June 16, 2010 (Chicago, Illinois) — In contrast to past advice to cancer patients to rest and avoid activity, the message now is to avoid inactivity. An expert panel convened by the American College of Sports Medicine (ACSM) has concluded that exercise training is safe during and after cancer treatments and can improve physical functioning, quality of life, and cancer-related fatigue.
The new ACSM guidelines urge cancer patients to be as physically active as possible both during and after treatment.
"The take-home message from the panel that put together the guidelines is to avoid inactivity during and posttreatment," said Kathryn Schmitz, PhD, MPH, associate professor of epidemiology and biostatistics at the University of Pennsylvania School of Medicine in Philadelphia. She presented the guidelines here at the American Society of Clinical Oncology 2010 Annual Meeting.
"Dozens of randomized controlled trials in a broad variety of patient populations have established the safety of exercise during treatment and the ability to go from being sedentary to completing 150 minutes of aerobic active over the course of even a single month," she said. "The risk–benefit leans heavily in the direction of getting patients moving and keeping them moving."
Exercise Oncology
Exercise is an area that is gaining an increasing awareness in the cancer literature, noted Jennifer A. Ligibel, MD, who moderated the session where the guidelines were presented. Dr. Ligibel is from the Dana-Farber Cancer Center in Boston, Massachusetts.
"If you had done a search between 1950 and 1979 using the words 'exercise/physical activity' and 'cancer,' you would have found 12 references," said Dr. Ligibel. "But in 2009, there were almost 500, more than almost all of the years put together."
A growing number of reports have now shown that physically active individuals are less likely to develop a number of common cancers, including breast, colon, advanced prostate, some gynecologic cancers, she pointed out. "Evidence is mounting and consistent. There has been a growing number of reports that physically active individuals tend to do better after being diagnosed with cancer."
Much of the data, she pointed out, have been in breast cancer.
Even with a growing number of reports, exercise oncology is a field that is still in its infancy, explained Lee Jones, PhD, scientific director of the Duke Center for Cancer Survivorship at Duke University in Durham, North Carolina. "There are only about 80 or so studies focused on exercise in cancer, and that really isn't all that many, especially when compared to cardiovascular disease — where there are about 3500."
Evidence So Far Is "Very Promising"
Dr. Jones, who was approached by Medscape Oncology for independent comment, pointed out that the evidence thus far is very promising. "Higher levels of physical activity are associated with a significant risk reduction in mortality in cancer patients," he said. "And the research to date provides a platform to launch second-generation studies."
The next level of studies will need to focus on why exercise is an effective intervention in cancer patients, if it does have an effect on recurrence, and if so, the underlying mechanism, Dr. Jones explained.
It is hoped that future research will answer such questions as how the effect can be maximized, what intensity is needed, and if exercise is effective for some types of tumors and not others, he said.
The ACSM guidelines indicate that exercise is safe during treatment, but Dr. Jones acknowledges that that can be a difficult time for patients. "They are battling many things during that time, such as side effects, and exercise might be a little tougher," he said. "But exercise programs can be modulated to suit the patient; that is the premise of personalized medicine."
An exercise program is also dependent on where the patient was in life before their diagnosis. "Were they sedentary, athletic, exercising occasionally?" he asked. "That information is important in shaping an exercise program."
Dr. Jones stated that he would like to get to the point where exercise becomes part of the standard of care for cancer. "It may eventually reach the same standard as it is in cardiac disease," he said. "I see us building this evidence base."
Specific Risks Need to Be Addressed
In creating the guidelines, the authors focused on the adult cancers and sites (breast, prostate, hematologic, colon, and gynecologic) where the most evidence has been assembled, and reviewed the literature for multiple health outcomes.
The panel found that even though there are specific risks associated with cancer treatment that need to be considered when patients embark on an exercise program, the evidence is consistent that it can improve aerobic fitness, muscular strength, quality of life, and fatigue in cancer survivors.
There are some general medical assessments that are recommended prior to exercise, explained Dr. Schmitz. For example, an evaluation for peripheral neuropathies and musculoskeletal morbidities secondary to treatment is recommended, regardless of the time since treatment.
"This doesn't have to be done in the physician's office or by a nurse," she said. "Fitness professionals can ask if there are any changes in balance or whether the person has noticed any tingling or changes in the kinds of symptoms that go along with peripheral neuropathies."
Evaluations of fracture risk are also recommended for individuals who have undergone hormonal therapy, and those with known metastatic disease to the bone will require evaluation to discern what is safe before starting exercise, she added. "Patients with metastatic disease to the bone should have medical clearance before starting an exercise program."
Individuals with known cardiac conditions, whether secondary to cancer or not, also require a medical assessment before starting exercise.
"There is recognition that there is always a risk that metastasis to the bone or cardiac toxicity secondary to cancer treatments will be undetected in a portion of our 12 million cancer survivors out there," Dr. Schmitz said. "This risk will vary widely across the population of survivors, and is likely quite low in the great majority of people who are diagnosed at an early stage and grade."
It is therefore recommended that fitness professionals consult with the patient's medical team to determine the likelihood of this risk, she added. "What we concluded as a body, in order to reduce barriers to physical activity programs, is that requiring medical assessment for metastatic disease and cardiotoxicity for all survivors prior to exercise is not recommended."
"We chose to do this because we felt that the small risk in a small body of patients is probably less than the risk of telling patients that they shouldn't exercise until they are cleared," she explained. "The risk of inactivity for the great majority of people at low risk is greater than the small risk of putting someone in harm's way."
Research Gaps Remain
The panel found that there is consistent evidence that exercise training can lead to improvements in aerobic fitness, muscular strength, quality of life, and fatigue in breast, prostate, and hematologic cancer patients and survivors, but that the data for colon and gynecologic cancers are still too limited to lead to conclusions.
Multiple research gaps remain in this field, the panel notes. These include a need for greater specificity with regard to the dose-response effects of specific modes of exercise training on specific end points and within a broader range of populations, such as survivors of colon and gynecologic cancers.
They also urge fitness trainers who work with cancer survivors to learn as much as possible about the specifics of the cancer diagnosis and treatment to make informed safe choices with regard to exercise testing and prescription.
Cancer diagnosis and treatment effects numerous body systems that are required for and affected by exercise training, they conclude, and "because cancer treatments are increasingly customized according to specific tumor characteristics, fitness professionals may benefit from contacting the medical treatment team for more precise information regarding the treatments received."
Dr. Schmitz and Dr. Ligibel have disclosed no relevant financial relationships.
American Society of Clinical Oncology (ASCO) 2010 Annual Meeting: Presented June 6, 2010.
The new ACSM guidelines urge cancer patients to be as physically active as possible both during and after treatment.
"The take-home message from the panel that put together the guidelines is to avoid inactivity during and posttreatment," said Kathryn Schmitz, PhD, MPH, associate professor of epidemiology and biostatistics at the University of Pennsylvania School of Medicine in Philadelphia. She presented the guidelines here at the American Society of Clinical Oncology 2010 Annual Meeting.
"Dozens of randomized controlled trials in a broad variety of patient populations have established the safety of exercise during treatment and the ability to go from being sedentary to completing 150 minutes of aerobic active over the course of even a single month," she said. "The risk–benefit leans heavily in the direction of getting patients moving and keeping them moving."
Exercise Oncology
Exercise is an area that is gaining an increasing awareness in the cancer literature, noted Jennifer A. Ligibel, MD, who moderated the session where the guidelines were presented. Dr. Ligibel is from the Dana-Farber Cancer Center in Boston, Massachusetts.
"If you had done a search between 1950 and 1979 using the words 'exercise/physical activity' and 'cancer,' you would have found 12 references," said Dr. Ligibel. "But in 2009, there were almost 500, more than almost all of the years put together."
A growing number of reports have now shown that physically active individuals are less likely to develop a number of common cancers, including breast, colon, advanced prostate, some gynecologic cancers, she pointed out. "Evidence is mounting and consistent. There has been a growing number of reports that physically active individuals tend to do better after being diagnosed with cancer."
Much of the data, she pointed out, have been in breast cancer.
Even with a growing number of reports, exercise oncology is a field that is still in its infancy, explained Lee Jones, PhD, scientific director of the Duke Center for Cancer Survivorship at Duke University in Durham, North Carolina. "There are only about 80 or so studies focused on exercise in cancer, and that really isn't all that many, especially when compared to cardiovascular disease — where there are about 3500."
Evidence So Far Is "Very Promising"
Dr. Jones, who was approached by Medscape Oncology for independent comment, pointed out that the evidence thus far is very promising. "Higher levels of physical activity are associated with a significant risk reduction in mortality in cancer patients," he said. "And the research to date provides a platform to launch second-generation studies."
The next level of studies will need to focus on why exercise is an effective intervention in cancer patients, if it does have an effect on recurrence, and if so, the underlying mechanism, Dr. Jones explained.
It is hoped that future research will answer such questions as how the effect can be maximized, what intensity is needed, and if exercise is effective for some types of tumors and not others, he said.
The ACSM guidelines indicate that exercise is safe during treatment, but Dr. Jones acknowledges that that can be a difficult time for patients. "They are battling many things during that time, such as side effects, and exercise might be a little tougher," he said. "But exercise programs can be modulated to suit the patient; that is the premise of personalized medicine."
An exercise program is also dependent on where the patient was in life before their diagnosis. "Were they sedentary, athletic, exercising occasionally?" he asked. "That information is important in shaping an exercise program."
Dr. Jones stated that he would like to get to the point where exercise becomes part of the standard of care for cancer. "It may eventually reach the same standard as it is in cardiac disease," he said. "I see us building this evidence base."
Specific Risks Need to Be Addressed
In creating the guidelines, the authors focused on the adult cancers and sites (breast, prostate, hematologic, colon, and gynecologic) where the most evidence has been assembled, and reviewed the literature for multiple health outcomes.
The panel found that even though there are specific risks associated with cancer treatment that need to be considered when patients embark on an exercise program, the evidence is consistent that it can improve aerobic fitness, muscular strength, quality of life, and fatigue in cancer survivors.
There are some general medical assessments that are recommended prior to exercise, explained Dr. Schmitz. For example, an evaluation for peripheral neuropathies and musculoskeletal morbidities secondary to treatment is recommended, regardless of the time since treatment.
"This doesn't have to be done in the physician's office or by a nurse," she said. "Fitness professionals can ask if there are any changes in balance or whether the person has noticed any tingling or changes in the kinds of symptoms that go along with peripheral neuropathies."
Evaluations of fracture risk are also recommended for individuals who have undergone hormonal therapy, and those with known metastatic disease to the bone will require evaluation to discern what is safe before starting exercise, she added. "Patients with metastatic disease to the bone should have medical clearance before starting an exercise program."
Individuals with known cardiac conditions, whether secondary to cancer or not, also require a medical assessment before starting exercise.
"There is recognition that there is always a risk that metastasis to the bone or cardiac toxicity secondary to cancer treatments will be undetected in a portion of our 12 million cancer survivors out there," Dr. Schmitz said. "This risk will vary widely across the population of survivors, and is likely quite low in the great majority of people who are diagnosed at an early stage and grade."
It is therefore recommended that fitness professionals consult with the patient's medical team to determine the likelihood of this risk, she added. "What we concluded as a body, in order to reduce barriers to physical activity programs, is that requiring medical assessment for metastatic disease and cardiotoxicity for all survivors prior to exercise is not recommended."
"We chose to do this because we felt that the small risk in a small body of patients is probably less than the risk of telling patients that they shouldn't exercise until they are cleared," she explained. "The risk of inactivity for the great majority of people at low risk is greater than the small risk of putting someone in harm's way."
Research Gaps Remain
The panel found that there is consistent evidence that exercise training can lead to improvements in aerobic fitness, muscular strength, quality of life, and fatigue in breast, prostate, and hematologic cancer patients and survivors, but that the data for colon and gynecologic cancers are still too limited to lead to conclusions.
Multiple research gaps remain in this field, the panel notes. These include a need for greater specificity with regard to the dose-response effects of specific modes of exercise training on specific end points and within a broader range of populations, such as survivors of colon and gynecologic cancers.
They also urge fitness trainers who work with cancer survivors to learn as much as possible about the specifics of the cancer diagnosis and treatment to make informed safe choices with regard to exercise testing and prescription.
Cancer diagnosis and treatment effects numerous body systems that are required for and affected by exercise training, they conclude, and "because cancer treatments are increasingly customized according to specific tumor characteristics, fitness professionals may benefit from contacting the medical treatment team for more precise information regarding the treatments received."
Dr. Schmitz and Dr. Ligibel have disclosed no relevant financial relationships.
American Society of Clinical Oncology (ASCO) 2010 Annual Meeting: Presented June 6, 2010.
Vitamin D May Cut Risk of Flu
June 17, 2010 — Vitamin D may reduce the incidence and severity of influenza and other infections of the upper respiratory tract, new research indicates.
Simple steps such as eating foods rich with vitamin D and getting more sunshine may help to reduce your chances of contracting flu and other similar illnesses, shows a study by scientists at Yale University School of Medicine and Greenwich Hospital in Connecticut.
"People in the South and West get more sun than in the North, which is good for them, because you get vitamin D from the sun," study researcher James R. Sabetta, MD, of the Yale University School of Medicine and Greenwich Hospital, Conn., tells WebMD. "It's not a panacea, but it helps."
Sabetta and his team of colleagues followed 198 healthy adults during the fall and winter of 2009-2010 to see if declining levels of vitamin D in the fall and winter could be a factor in the seasonal increased prevalence of respiratory viral infections, such as flu.
The study shows people who maintain vitamin D blood levels of 38 nanograms per milliliter or more are less likely to get viral infections such as flu than people with less in their blood.
Of 18 people who maintained that level during the study period, only three developed viral infections.
But of the 180 other participants with less vitamin D in their blood, 81(45%), did get sick with viral infections.
And those with higher levels of vitamin D also experienced a marked reduction in the number of days they were ill, Sabetta tells WebMD.
In addition to getting more sun and consuming milk and foods with vitamin D, he recommends supplements, especially for people in areas with less sunlight and for those who spend daylight hours in darker, indoor environments.
"If you have a level of 38, your risk is down 50%," he tells WebMD. "A lot of people don't have an adequate level, and 38 is a little above what you should have to be considered in the sufficient range. There are a billion people worldwide with levels below 30."
And 30 is considered "sufficient," he says.
Watching for Signs of Illness
Participants in the study had blood samples drawn monthly using a sophisticated technique to accurately measure vitamin D levels. They didn't know that vitamin D was being measured, and even investigators didn't know until the end of the study.
All participants were asked to report signs of illness, such as nasal congestion, sore throat, cough with or without fever, chills, fatigue and general malaise.
Those reporting any symptoms were seen the same day at the study site by one of the infectious disease investigators.
People in the study kept a diary of symptoms and were called every one to three days during the illness to review any signs of symptoms until they were better. The investigators recorded the duration of each symptom, the total duration of the illness, and any antimicrobials administered.
Sabetta says the findings suggest that supplementing vitamin D to achieve a blood level of 38 nanograms per milliliter or higher could result in a significant health benefit by reducing odds of contracting viral infections of the respiratory tract.
But he says more studies are needed to determine the efficacy of vitamin D supplementation in the prevention of infections, including influenza.
The researchers conclude that the lower levels of vitamin D seen during the winter in temperate climates may contribute to the prevalence of influenza in colder months.
The findings, Sabetta says, have significant implications for public health and also may explain the seasonality of certain infections, and also the higher morbidity and mortality of such illnesses in people who are predisposed to lower concentrations of vitamin D.
Sabetta says vitamin D has known effects on the immune system, and the study reinforces the association between vitamin D deficiency and susceptibility to infections of the respiratory tract.
The study is published online in the journal Plos ONE.
Vitamin D levels depend "on how big you are, your skin color, your diet and how much sun exposure you get," Sabetta tells WebMD. "Individuals should get their vitamin D levels checked. If you are gardening a lot, you probably are fine, but people in an office all day may need supplements."
SOURCES:
News release, Greenwich Hospital.
Sabetta, J. Plos ONE, June 16, 2010.
James Sabetta, MD, Yale University School of Medicine, Greenwich Hospital, Greenwich, Conn.
Simple steps such as eating foods rich with vitamin D and getting more sunshine may help to reduce your chances of contracting flu and other similar illnesses, shows a study by scientists at Yale University School of Medicine and Greenwich Hospital in Connecticut.
"People in the South and West get more sun than in the North, which is good for them, because you get vitamin D from the sun," study researcher James R. Sabetta, MD, of the Yale University School of Medicine and Greenwich Hospital, Conn., tells WebMD. "It's not a panacea, but it helps."
Sabetta and his team of colleagues followed 198 healthy adults during the fall and winter of 2009-2010 to see if declining levels of vitamin D in the fall and winter could be a factor in the seasonal increased prevalence of respiratory viral infections, such as flu.
The study shows people who maintain vitamin D blood levels of 38 nanograms per milliliter or more are less likely to get viral infections such as flu than people with less in their blood.
Of 18 people who maintained that level during the study period, only three developed viral infections.
But of the 180 other participants with less vitamin D in their blood, 81(45%), did get sick with viral infections.
And those with higher levels of vitamin D also experienced a marked reduction in the number of days they were ill, Sabetta tells WebMD.
In addition to getting more sun and consuming milk and foods with vitamin D, he recommends supplements, especially for people in areas with less sunlight and for those who spend daylight hours in darker, indoor environments.
"If you have a level of 38, your risk is down 50%," he tells WebMD. "A lot of people don't have an adequate level, and 38 is a little above what you should have to be considered in the sufficient range. There are a billion people worldwide with levels below 30."
And 30 is considered "sufficient," he says.
Watching for Signs of Illness
Participants in the study had blood samples drawn monthly using a sophisticated technique to accurately measure vitamin D levels. They didn't know that vitamin D was being measured, and even investigators didn't know until the end of the study.
All participants were asked to report signs of illness, such as nasal congestion, sore throat, cough with or without fever, chills, fatigue and general malaise.
Those reporting any symptoms were seen the same day at the study site by one of the infectious disease investigators.
People in the study kept a diary of symptoms and were called every one to three days during the illness to review any signs of symptoms until they were better. The investigators recorded the duration of each symptom, the total duration of the illness, and any antimicrobials administered.
Sabetta says the findings suggest that supplementing vitamin D to achieve a blood level of 38 nanograms per milliliter or higher could result in a significant health benefit by reducing odds of contracting viral infections of the respiratory tract.
But he says more studies are needed to determine the efficacy of vitamin D supplementation in the prevention of infections, including influenza.
The researchers conclude that the lower levels of vitamin D seen during the winter in temperate climates may contribute to the prevalence of influenza in colder months.
The findings, Sabetta says, have significant implications for public health and also may explain the seasonality of certain infections, and also the higher morbidity and mortality of such illnesses in people who are predisposed to lower concentrations of vitamin D.
Sabetta says vitamin D has known effects on the immune system, and the study reinforces the association between vitamin D deficiency and susceptibility to infections of the respiratory tract.
The study is published online in the journal Plos ONE.
Vitamin D levels depend "on how big you are, your skin color, your diet and how much sun exposure you get," Sabetta tells WebMD. "Individuals should get their vitamin D levels checked. If you are gardening a lot, you probably are fine, but people in an office all day may need supplements."
SOURCES:
News release, Greenwich Hospital.
Sabetta, J. Plos ONE, June 16, 2010.
James Sabetta, MD, Yale University School of Medicine, Greenwich Hospital, Greenwich, Conn.
Subscribe to:
Posts (Atom)